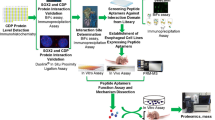
Abstract
Background
Meningiomas are the most common type of intracranial tumor, accounting for between 24 and 30 % of primary intracranial tumors. Thus far, no biomarkers exist to reliably predict the clinical outcome of meningiomas. A previous genome-wide methylation analysis revealed that HOXA9 is one of the most functionally relevant biomarkers. In this study, we have examined whether HOXA9 is a potential therapeutic target in meningiomas, using HXR9, a peptide inhibitor of the interaction between HOXA9 and its cofactor PBX.
Methods
We determined the expression level of HOXA9 in human meningiomas, meningioma cell lines, and normal brain tissue. Meningioma in culture and in subcutaneous tumors was treated with HXR9. We also examined the disruption of HOXA9/PBX dimers.
Results
We first confirmed that HOXA9 is highly expressed in meningiomas, but not in normal brain tissue. The HXR9 peptide blocks the binding of HOXA9 to PBX, leading to an alteration of DNA binding, and subsequent regulation of their target genes. HXR9 markedly inhibited the growth of meningioma cells and subcutaneous meningeal tumors.
Conclusion
There is no effective chemotherapy for meningiomas at present, and targeting the HOXA9/PBX interaction may represent a novel treatment option for this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are the most common of the intracranial tumors and account for between 24 and 30 % of primary intracranial tumors. Most meningiomas are curable by surgical resection; however, some meningiomas with benign pathological findings pursue a clinically malignant course [2, 4, 19]. Thus far, no biomarkers exist to reliably predict the clinical outcome of meningiomas. Previously, we classified histologically benign and malignant meningiomas into 3 subgroups, based on a genome-wide methylation analysis. Our classifications were found to correlate well with clinical recurrence/progression, but not with more classical predictors. We demonstrated that HOXA9 was one of the most functionally relevant in the clinically malignant cluster that could act as biomarkers for a poor prognosis [16].
The development and maintenance of cellular identity is vital for normal organ function in both embryonic and adult tissues. Homeobox (HOX) genes are major regulators of the animal body plan, as they help to confer cell and tissue identity. Mammals have 39 HOX genes which are divided into 4 groups (A–D), according to their locus [8, 12], with HOXA located at 7p15.3, HOXB at 17q21.3, HOXC at 12q13.3, and HOXD at 2q31 [6]. In normal early development, these genes determine the identities of sequential zones along the main body axis and define the identity of cells and tissues, including those of the developing nervous system [8, 11, 14].
Some HOX genes have distinct functions in specific contexts, primarily in embryonic tissues, while many other HOX genes have overlapping or redundant functions, especially in adult tissues. Although HOX genes are generally expressed at a low level in most adult cells, a number of studies have now shown that there is frequently a profound de-regulation of HOX genes in various cancers and that this confers enhanced survival and proliferation [10, 20, 24, 29]. Many of these HOX functions are dependent on their interaction with a common set of cofactors, including the pre-B cell leukemia transcription factor (PBX). PBX modifies HOX function, including DNA binding specificity, and is therefore crucial in the correct regulation of target genes. [18, 22]. This interaction is mediated by the binding of a small tryptophan-containing motif within the HOX protein to a hydrophobic pocket formed by PBX/PBC proteins. This motif is known as the PBC interaction domain (PID) and includes a “YPWM” motif [18].
A novel peptide, HXR9, consists of PID and 7 arginine repeat residues. The arginine repeat structure allows HXR9 to penetrate the cell membrane and move into the cytoplasm and nucleus to bind with PBX, leading to an inhibitory interaction with the HOX/PBX pocket [7, 23–26, 28].
In this study, we extend our previous finding that HOXA9 methylation is a biomarker in meningiomas and examine whether HOXA9 could also be a potential therapeutic target.
Materials and methods
Cell lines
The human meningioma cell lines IOMM-Lee and HKBMM were kindly provided by Drs. Anita Lai (University of California at San Francisco, CA) and Shinichi Miyatake (Osaka Medical University, Osaka, Japan), respectively. All cell lines were maintained in Dulbecco’s modified Eagle medium containing 10 % fetal bovine serum (FBS) and 1 % penicillin/streptomycin. Cell lines were grown at 37 °C in a humidified atmosphere of 5 % CO2.
Antibodies
Mouse anti-Pbx1a/2/3a antibody (359.15, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-HoxA9 antibody (2A11-2D2, Sigma-Aldrich, St. Louis, MO), and rabbit anti-β-actin (Sigma-Aldrich, St. Louis, MO) were used.
Peptides
The HXR9 (WYPWMKKHHRRRRRRRRR-) and control CXR9 (WYPAMKKHHRRRRRRRRR) peptides were synthesized by ThermoFisher Scientific (Waltham, MA).
Sample collection and RNA extraction
Tumor specimens for molecular genetic analysis were obtained from 23 meningioma patients who underwent surgical procedures at Nagoya University Hospital or affiliated hospitals. The analyses performed in this study were approved by the institutional ethics committee of Nagoya University, and all patients who registered for this study provided their written informed consent. All tumors were histologically verified according to WHO 2007 guidelines; 16 patients had grade I meningioma, 6 had grade II meningioma, and 1 had grade III meningioma. RNA purification was performed using the standard TRIzol (Invitrogen, Carlsbad, CA) method.
Quantitative analysis of HOXA9 and cFos mRNA expression
Total RNA was extracted from 23 tumors, 2 cell lines, and from normal whole brain (Human Total RNA Master Panel II; Takara Bio, Otsu, Japan), and first-strand cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany). The cDNA product was used in qRT-PCR for the quantitation of HOXA9, cFos, and GAPDH mRNA levels. The primers for this assay were purchased from Roche Diagnostics (Indianapolis, IN). The sequences of the primers and probe were as follows: HOXA9 forward (5′-GCTTGTGGTTCTCCTCCAGT-3′) and HOXA9 reverse (5′-CCAGGGTCTGGTGT-TTTGTA-3′) and cFos forward (5′-GTGGCTCTGAGACAGCCCGC-3′ and reverse (5′-GCGTGGGTGAGCTGAGCGAG-3′). Quantitative real-time PCR (qPCR) was performed using the Light Cycler 480 system and Light Cycler 480 SYBR Green I Master Mix (Roche). The sequences of the primers used to detect GAPDH mRNA were as follows: GAPDH forward primer (5′-AGCCACATCGCTCAGACAC-3′) and GAPDH reverse primer (5′-GCCCAATACGACCAAATCC-3′). The GAPDH probe was purchased from the Roche Human Probe Library (no. 60). The expression was normalized to that of GAPDH in each sample.
Protein extracts and Western blot analysis
Cells were lysed, and 10 μL lysate was applied to each well of 10 % Mini-PROTEAN TGX Gels (BIO-RAD, Hercules, CA). Separated proteins were electro-transferred onto PVDF membrane (Hybond-P, GE Healthcare, Buckinghamshire, UK). Western blots were performed using anti-Pbx 1a/2/3a and anti-HoxA9 antibodies. ECL Western Blotting Detection Reagents (GE Healthcare) were used for signal detection, according to the manufacturer’s protocols.
Protein interaction assays
For immunoprecipitation analyses, cells were seeded onto 25 cm2 plates, and either HXR9 or CXR9 was administered, with a final concentration of 40 μM. The cells were lysed using TNE buffer (150 mM NaCl, 35 mM Tris–HCl, 1 % NP-40, 1 mM EDTA, 10 μg/mL aprotinin) at 4 °C. The lysate was used for immunoprecipitation, which was performed with the anti-Pbx 1a/2/3a antibody and protein G agarose (Thermo Scientific, Rockford, IL). The immunoprecipitated samples were analyzed by Western blotting, as previously described.
Immunocytochemistry
IOMM-Lee cells were seeded onto microscope cover slips in a 12-well plate and grown for 48 h. Then, either HXR9 or CXR9 was added, to a final concentration of 40 μM. After 48 h, cells were fixed with PBS containing 3.7 % (v/v) formaldehyde for 30 min at room temperature and subjected to immunocytochemical analysis, as previously described, using as the specific primary antibodies anti-Pbx 1a/2/3a or anti-HoxA9, used according to the manufacturer’s instructions. Cells were then incubated for 1 h at room temperature. After incubation, Alexa Fluor 488, 546, and DAPI fluorescence antibodies (Invitrogen Molecular Probes, Eugene, Oregon) were added to the samples. Immunofluorescence was observed using a laser scanning confocal microscope (OLYMPUS FV1000-D, Olympus, Tokyo, Japan).
Cell growth assay
Cells were seeded in 96-well plates at a density of 1 × 103 cells/well. After 12 h, the medium was changed, and a cell counting Kit-8 (DOJINDO, Osaka, Japan) was used in accordance with manufacturer’s protocols, the absorbance was measured pre-treatment, and HXR9 or CXR9 was then added to the culture medium at a final concentration of 0, 20, or 40 μM. After a 48-h exposure to the peptides, the absorbance was again measured using a Multilabel Reader 2030 ARVO X4 (PerkinElmer, Waltham, MA).
Cell cycle analysis
Cell cycle analysis by propidiumiodide staining of the cells treated with HXR9 or CXR9 was assessed using a FACS Calibur apparatus after 48-h exposure of the peptides.
Xenograft in vivo assay
IOMM-Lee cells (106 cells) were injected into the flanks of bulb-c nu/nu nude mice. Treatment was started 7 days after the induced tumor had grown to a volume of approximately 100 mm3. The treatment consisted of 30 mg/kg HXR9 or CXR9 i.v. administered on days 7, 9, 13, 16, and 19. The optimal treatment dose and schedule were selected based on our previous experiments [20–23]. Tumor length (a) and width (b) were measured in situ with digital calipers daily, and tumor volumes were calculated according to the following formula: V (mm3) = a × b2/2.
Histology
Immunohistochemical analysis was performed as previously described [31]. Briefly, after antigen retrieval with autoclave in 0.01 M citrate buffer pH6.0 at 121 °C for 10 min, Ki-67 was stained with the MIB-1 antibody (Dako, Glostrup, Denmark). For each immunostained slide, the percentage of positively stained cells on a given slide was evaluated.
Statistical analysis
Data are expressed as mean ± SEM. Comparison of treatments against controls was made using one-way ANOVA followed by Fisher’s LSD post hoc test with SPSS version 19 (IBM, Chicago, IL). P values of <0.05 were considered significant.
Results
HOXA9 is aberrantly expressed in meningiomas
The expression of HOXA9 in 2 meningioma cell lines, IOMM-Lee and HKBMM, was detected by conventional RT-PCR, but was not found in healthy adult brain (Fig. 1a). Quantitative RT-PCR demonstrated that HOXA9 was abundantly expressed in the majority of meningiomas studied, but was not associated with the WHO tumor grading (Fig. 1b).
HOXA9 is aberrantly expressed in meningiomas, but not in normal brain tissue. a The expression of HOXA9 in 2 meningioma cell lines, IOMM-Lee and HKBMM was detected by conventional RT-PCR, but was not found in healthy adult brain (arrow head). b Quantitative RT-PCR demonstrated that HOXA9 was highly expressed in a large number of meningiomas, but was not associated with the WHO tumor grading
The novel HOXA9-targeting peptide HXR9 inhibits the proliferation of meningioma cells
Given the over-expression of HOXA9 in meningioma, we treated 2 representative meningioma cell lines, IOMM-Lee and HKBMM cells, with either HXR9 or with CXR9, a control peptide containing a single amino acid substitution. HXR9 significantly inhibited the proliferation of both of these cell lines in a dose-dependent manner (Fig. 2a). While previous reports found that HXR9 induced apoptosis through caspase activation [23, 24], cell cycle analysis demonstrated that no apoptotic cells were observed in our study (Fig. 2b). Thus, the effect of HXR9 seemed to be cytostatic in meningioma cells.
a The meningioma cell lines IOMM-Lee and HKBMM were both treated with either HXR9 or with a control peptide, CXR9. HXR9 significantly inhibited the proliferation of both of these cell lines in a dose-dependent manner. b Cell cycle analysis by propidiumiodide staining of the cells treated with HXR9 or CXR9 was assessed using a FACS Calibur apparatus after 48-h exposure of the peptides. Few apoptotic cells were observed in both cell lines
HXR9 inhibits binding to PBX and translocation into the nucleus
Protein lysates from IOMM-Lee cells treated with either HRX9 or CXR9 were immunoprecipitated with the anti-PBX antibody, and precipitation was assessed by Western blotting with the anti-HOXA9 antibody. HRX9 inhibited binding of HOXA9 to PBX, but the control peptide did not (Fig. 3a). Additionally, although HXR9 did not reduce the expression of HOXA9 (Figs. 3b, 4), PBX was observed in the cytoplasm, but not in the nucleus of the HXR9-treated cells (Fig. 3b). Thus, HXR9 inhibited the translocation of HOXA9 into the nucleus by preventing its binding to PBX.
HXR9 does not up-regulate HOXA9 and cFos
Previous studies have suggested that an increase in Fos transcription could induce apoptosis [9, 15, 27] and that the up-regulation of cFos is a key event in HXR9-mediated apoptosis [24]. In contrast, in this study, HRX9 treatment did not induce the expression of cFos or up-regulate HOXA9. This finding was consistent with our observation that HXR9 did not induce apoptosis, but had an anti-proliferative effect in meningioma cells.
HXR9 slows tumor growth in vivo
Subcutaneous tumors induced in nude mice were treated intravenously with either HXR9 or CRX9 on days 7, 9, 13, 16, and 19. Mice administered HXR9 exhibited a significant tumor volume reduction from day 14, compared to CRX9-treated mice (Fig. 5a). There was no significant difference in the histology and Ki-67 labeling index between CXR9 and HXR9 treatments in vivo, except for tumor size at day 21.
a HXR9 retards tumor growth in vivo. Subcutaneous tumors induced in nude mice were treated intravenously with either HXR9 or CRX9 on days 7, 9, 13, 16, and 19. Mice administered HXR9 exhibited a significant tumor volume reduction from day 14, compared to CRX9-treated mice. b There was no significant difference in the histology and Ki-67 labeling index (1.5 and 0.9 %, respectively) between CXR9 and HXR9 treatments in vivo at day 21
Discussion
The role of HOX genes in early development is highly conserved. They may also play a common role in malignant cells and are frequently dysregulated in various malignancies [1, 5, 21, 28, 30]. HOX genes confer specific identities on embryonic cells and may also influence the specific phenotype of the cancer. There are a large number of HOX genes, and generally, the specific function of each remains unclear.
However, we have previously demonstrated that methylation of the HOXA9 gene is associated with a poor prognosis in meningioma. In the current investigation, we showed that HOXA9 is highly expressed in meningiomas, but not in normal brain tissue. The expression level of HXA9 and tumor grade of meningioma were not correlated as the number of each grade was limited. The target genes of HOXA9 were recently identified. TGF-β2 is transcriptionally activated by HOXA9 in cancer-associated fibroblasts, which results in the growth of ovarian cancer via the paracrine actions of CXCL12, IL-6, and VEGF-A [17]. Together with the cofactor Meis1 and histone modification, HOXA9 transcriptionally activates a network of proto-oncogenes including Erg, Flt3, Lmo2, Myb, and Sox4 [13]. Among these genes, the expression of Erg is increased in 40 % of meningiomas [32], and the downregulation of Flt3 reduces the survival and migration of meningioma cells [3]. One challenge in targeting HOX genes is that they generally have a high degree of functional redundancy, which can mask the specific contribution of the individual genes in a malignant phenotype. Nevertheless, HOXA9-targeting treatments may be promising in meningiomas that express large amounts of HOXA9.
Therefore, a post-translational approach may prove to be more specific. The HRX9 peptide blocks the binding of HOXA9 to PBX, leading to an alteration of DNA binding, and subsequent regulation of their target genes. HRX9 markedly inhibited the growth of meningioma cells and subcutaneous meningeal tumors. A change of cFos expression was previously shown to be responsible for the induction of apoptosis [24]; however, its expression was not changed after HRX9 treatment, probably because the effect of HRX9 was anti-proliferative rather than apoptotic.
Conclusion
There is no effective chemotherapy for meningiomas at present. Targeting the HOXA9/PBX interaction may represent a novel treatment option for this disease.
References
Abe M, Hamada J, Takahashi O, Takahashi Y, Tada M, Miyamoto M, Morikawa T, Kondo S, Moriuchi T (2006) Disordered expression of HOX genes in human non-small cell lung cancer. Oncol Rep 15:797–802
Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjani S, Martuza RL, Curry WT Jr, Barker FG (2009) Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 64:56–60
Andrae N, Kirches E, Hartig R, Haase D, Keilhoff G, Kalinski T, Mawrin C (2012) Sunitinib targets PDGF-receptor and Flt3 and reduces survival and migration of human meningioma cells. Euro J Cancer 48:1831–1841
Backer-Grøndahl T, Moen BH, Torp SH (2012) The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol 5:231
Cantile M, Procino A, D’Armiento M, Cindolo L, Cillo C (2003) HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis. J Cell Physiol 194:225–236
Costa BM, Smith JS, Chen Y, Chen J, Phillips HS, Aldape KD, Zardo G, Nigro J, James CD, Fridlyand J, Reis RM, Costello JF (2010) Reversing HOXA9 oncogene activation by PI3 K inhibition: epigenetic mechanism and prognostic significance in human glioblastoma. Cancer Res 70:453–462
Daniels TR, Neacato II, Rodriguez JA, Pandha HS, Morgan R, Penichet ML (2010) Disruption of HOX activity leads to cell death that can be enhanced by the interference of iron uptake in malignant B cells. Leuk Off J Leuk Soc Am Leuk Res Fund U.K 24:1555–1565
Durston AJ (2011) Hox homeotic selector genes: key regulators of embryogenesis. WebmedCentral EMBRYOLOGY 2:WMC002573
Eichhorst ST, Muller M, Li-Weber M, Schulze-Bergkamen H, Angel P, Krammer PH (2000) A novel AP-1 element in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol Cell Biol 20:7826–7837
Eklund EA (2007) The role of HOX genes in malignant myeloid disease. Cur Opin Hematol 14:85–89
Grier D, Thompson A, Kwasniewska A, McGonigle G, Halliday H, Lappin T (2005) The pathophysiology of HOX genes and their role in cancer. J Pathol 205:154–171
Hoegg S, Meyer A (2005) Hox clusters as models for vertebrate genome evolution. Trends Genet 21:421–424
Huang Y, Sitwala K, Bronstein J, Sanders D, Dandekar M, Collins C, Robertson G, MacDonald J, Cezard T, Bilenky M, Thiessen N, Zhao Y, Zeng T, Hirst M, Hero A, Jones S, Hess JL (2012) Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood 119:388–398
Iimura T, Pourquié O (2007) Hox genes in time and space during vertebrate body formation. Dev Growth Differ 49:265–275
Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR (1998) DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell 1:543–551
Kishida Y, Natsume A, Kondo Y, Takeuchi I, An B, Okamoto Y, Shinjo K, Saito K, Ando H, Ohka F (2012) Epigenetic subclassification of meningiomas based on genome-wide DNA methylation analyses. Carcinogenesis 33:436–441
Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, Naora H (2012) HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Investig 122:3603–3617
Laurent A, Bihan R, Omilli F, Deschamps S, Pellerin I (2008) PBX proteins: much more than Hox cofactors. Int J Dev Biol 52:9
Maier H, Öfner D, Hittmair A, Kitz K, Budka H (1992) Classic, atypical, and anaplastic meningioma: three histopathological subtypes of clinical relevance. J Neurosurg 77:616–623
McIntyre DC, Rakshit S, Yallowitz AR, Loken L, Jeannotte L, Capecchi MR, Wellik DM (2007) Hox patterning of the vertebrate rib cage. Development 134:2981–2989
Miller GJ, Miller HL, van Bokhoven A, Lambert JR, Werahera PN, Schirripa O, Lucia MS, Nordeen SK (2003) Aberrant HOXC expression accompanies the malignant phenotype in human prostate. Cancer Res 63:5879–5888
Moens CB, Selleri L (2006) Hox cofactors in vertebrate development. Dev Biol 291:193–206
Morgan R, Boxall A, Harrington KJ, Simpson GR, Gillett C, Michael A, Pandha HS (2012) Targeting the HOX/PBX dimer in breast cancer. Breast Cancer Res Treat 136:389–398
Morgan R, Pirard PM, Shears L, Sohal J, Pettengell R, Pandha HS (2007) Antagonism of HOX/PBX dimer formation blocks the in vivo proliferation of melanoma. Cancer Res 67:5806
Morgan R, Plowright L, Harrington KJ, Michael A, Pandha HS (2010) Targeting HOX and PBX transcription factors in ovarian cancer. BMC Cancer 10:89
Plowright L, Harrington K, Pandha H, Morgan R (2009) HOX transcription factors are potential therapeutic targets in non-small-cell lung cancer (targeting HOX genes in lung cancer). Br J Cancer 100:470–475
Rich KA, Zhan Y, Blanks JC (1997) Aberrant expression of c-Fos accompanies photoreceptor cell death in the rd mouse. J Neurobiol 32:593–612
Shears L, Plowright L, Harrington K, Pandha HS, Morgan R (2008) Disrupting the interaction between HOX and PBX causes necrotic and apoptotic cell death in the renal cancer lines CaKi-2 and 769-P. J Urol 180:2196–2201
Shears L, Plowright L, Harrington K, Pandha HS, Morgan R (2008) Disrupting the interaction between HOX and PBX causes necrotic and apoptotic cell death in the renal cancer lines CaKi-2 and 769-P. J Urol 180:2196–2201
Waltregny D, Alami Y, Clausse N, de Leval J, Castronovo V (2002) Overexpression of the homeobox gene HOXC8 in human prostate cancer correlates with loss of tumor differentiation. Prostate 50:162–169
Watanabe R, Nakasu Y, Tashiro H, Mitsuya K, Ito I, Nakasu S, Nakajima T (2011) O6-methylguanine DNA methyltransferase expression in tumor cells predicts outcome of radiotherapy plus concomitant and adjuvant temozolomide therapy in patients with primary glioblastoma. Brain Tumor Pathol 28:127–135
Yaskiv O, Rubin BP, He H, Falzarano S, Magi-Galluzzi C, Zhou M (2012) ERG protein expression in human tumors detected with a rabbit monoclonal antibody. Am J Clin Pathol 138:803–810
Acknowledgments
This work was supported by a Grant-in-Aid (B) for Scientific Research from the Japan Society for the Promotion of Science (AN).
Conflict of interest
The authors declare that no conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ando, H., Natsume, A., Senga, T. et al. Peptide-based inhibition of the HOXA9/PBX interaction retards the growth of human meningioma. Cancer Chemother Pharmacol 73, 53–60 (2014). https://doi.org/10.1007/s00280-013-2316-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2316-5