Abstract
Purpose
The potential synergy of modulating platinum-induced DNA damage by combining the proteasome inhibitor bortezomib with oxaliplatin was studied in patients with solid tumors, with special attention to avoidance of cumulative neurotoxicity (NT).
Patients and methods
In a 3 + 3 dose escalation design, patients received bortezomib at 1.0–1.5 mg/m2 on days 1 and 4 and oxaliplatin at 60–85 mg/m2 on day 1 of a 14-day cycle. NT assessments were performed at the start of every two cycles. Oxaliplatin pharmacokinetics (PK) were determined pre- and post-bortezomib.
Results
Thirty patients were enrolled with 25 (11 men, 14 women) fully evaluable for NT assessments at cycle 2. The median age was 56 years (range 35–74 years); median number of cycles received 2 (range 1–10). At dose levels 2–5 (B 1.3 mg/m2), patients manifested NT grades 3 and 4 at a median 3.4 cycles (range 2–9 cycles): 3 had ataxia (one also with sensory neuropathy or neurogenic hypotension, respectively) and 3 had just sensory neuropathy. A 6th dose-level reducing bortezomib to 1.0 mg/m2 with oxaliplatin 85 mg/m2) was explored and no NT or dose limiting toxicities were noted among 7 evaluable patients (5 receiving two or more cycles). Four patients experienced a partial response—one with platinum-resistant ovarian cancer, another with gastroesophageal cancer, another with ampulla of Vater carcinoma, and a patient with cholangiocarcinoma. PK studies at dose levels 1 and 2 showed greater mean ultrafiltrable platinum when oxaliplatin was dosed after bortezomib.
Conclusions
Bortezomib 1.0 mg/m2 × 2 every 14 days combines safely with oxaliplatin. At higher doses, cumulative NT (i.e., cerebellar signs and sensory neuropathy) occurs at an accelerated pace perhaps from a PK interaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bortezomib is the first proteasome inhibitor approved by the FDA, targeting the 20S subunit of the proteasome [1]. It has become widely used in the treatment of hematologic malignancies—especially multiple myeloma [2, 3]. In our previous phase I trial performed under the aegis of the Cancer Therapy Evaluation Program, National Cancer Institute, a unique day 1 and 4 every-two-week schedule determined the recommended phase II dose to be 1.75 mg/m2/dose [4]. On this schedule, dose-limiting toxicity (DLT) only occurred if a 2 mg (flat dose) was exceeded. Above this dose, patients began to encounter painful neuropathy, diarrhea, and thrombocytopenia. Notably, proteasome 20S activity is inhibited in a dose-dependent manner with increased bortezomib doses from 1 mg/m2 dose level to the highest dose level explored, 1.9 mg/m2.
When used as a single agent in solid tumors, bortezomib has not shown major activity. Alone, bortezomib had modest activity, but considerable intolerance in an ovarian cancer study by the Gynecology Oncology Group (GOG) [5] and interest in further studies waned. Furthermore, interest in the use of bortezomib in colon cancer (by hypothetical effects in reversing NFkB activation) declined after lack of activity as single agent, as well as little apparent contribution in combination with chemotherapy [6]. Nevertheless, the potential for drug synergy has been highlighted in preclinical studies of proteasome inhibition, suggesting interference with chemotherapy-resistance pathways mediated by the activation of NFkB [7]. Pursuing a combination of bortezomib and platinum was bolstered following demonstration that the copper transporter CTR1, which mediates cisplatin cellular uptake, was down-regulated by ubiquitination and proteasomal degradation—leading to higher intracellular platinum levels [8]. Interest was further increased by a phase I study of women with gynecologic cancer treated with carboplatin and bortezomib that reported 47 % objective responses among 36 platinum-pretreated patients and no cumulative neurotoxicity (NT) [9].
Providing proteasome inhibition below a dose known to be associated with neuropathy might permit safe, cautious exploration of its combined administration with platinum drugs, including one that is known to be neurotoxic such as oxaliplatin. If a safe combination could be developed, it might prove useful subsequently in the treatment of colorectal and other GI cancer patients, who have less exposure to taxanes and other drugs that contribute to the intolerance of pretreated ovarian cancer patients. We recognized that neurological toxicity is a major DLT for oxaliplatin: a peripheral sensory neuropathy characterized by dysesthesias and/or distal paresthesias often triggered or exacerbated by cold. The neuropathy is a result of cumulative doses: at a dose of 85 mg/m2, 10 % of patients will develop at least grade 3 NT after nine cycles, whereas it is seen in 25 % of patients after twelve cycles, and in 50 % after fourteen cycles (cumulative dose of >1,000 mg/m2) [10]. Following discontinuation of treatment, the neurologic symptoms do improve in >90 % of patients over the next 12 months [10–12]. Accordingly, we embarked in this phase I study to find a tolerable dose of bortezomib in our d1 and 4 q2w schedule that could be safely combined with oxaliplatin, with a special focus on NT assessment.
Patients and methods
Patients 18 years old or older with histologically confirmed cancers were eligible after the failure of standard therapy or if no other known effective treatment was available. Eastern Cooperative Oncology Group (ECOG) performance status ≤2, hematologic parameters including absolute neutrophil count (ANC) ≥1,500/mm3 and platelets ≥100,000/mm3; liver function studies within normal institutional limits, and serum creatinine ≤1.5 mg/dL were required. Recovery from the effects of prior chemotherapy and radiation therapy (maximum of grade 1 non-hematologic toxicity), with a minimum of 3 (6 for mitomycin C and nitrosoureas) weeks to elapse since last chemotherapy and/or radiotherapy was required. Known brain metastases and any baseline peripheral neuropathy were exclusions.
The protocol was reviewed and approved by the Cancer Therapy Evaluation Program of the National Cancer Institute (Bethesda, MD, USA) along with the Protocol Review and Monitoring Committees of the NYU Cancer Institute and Institutional Research Boards of the New York University School of Medicine. All participating patients signed an informed consent form reviewed and approved by the Cancer Therapy Evaluation Program and the local institutional review boards. The study opened in July 2004.
Bortezomib was administered as an IV bolus on days 1 and 4 of weeks 1 and 3 every 4 weeks. Oxaliplatin was administered intravenously as a 2-h infusion on day 1 or weeks 1 and 3 every 4 weeks. All were pretreated with HT3 anti-emetics; calcium gluconate 1 g and magnesium sulfate 1 g in 100 ml of D5W over 15 min pre- and post-oxaliplatin infusion as previously reported to abrogate acute and chronic neuropathy [13, 14]. Loperamide was provided in the event of diarrhea. Hematologic growth factors were not administered prophylactically.
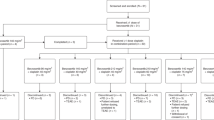
The dose escalation schedule for bortezomib/oxaliplatin (mg/m2) was as shown in the initial 5 steps as follows: 1/60; 1.3/60; 1.3/75; 1.3/85; 1.5/75, and then an additional level was explored, 1/85, because of observed NT. All toxicities other than sensory neuropathy were graded according to the NCI Common Toxicity Criteria, version 2.0. Sensory neuropathy was graded according to the oxaliplatin sensory neuropathy scale (Table 1); similar scales have been published in the oxaliplatin literature [15, 16]. DLT was defined as grade 3 or 4 diarrhea despite loperamide therapy, grade 4 hematologic toxicity, febrile neutropenia or grade 3 or 4 non-hematologic toxicity occurring during the first 28 days of therapy. Peripheral blood complete blood counts and chemistries were performed every 2 weeks, and tumor response was assessed every 8 weeks using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, version 1 [17]. In addition to standard clinical evaluations, NT was comprehensively evaluated every 28 days and at the onset of any clinical symptoms using the neuropathy assessment protocol. Three assessable patients were to be entered at each dose level, which were to be expanded by three additional patients upon recording a DLT. If two or more patients developed DLTs, then the next lower dose level would be expanded. The recommended phase II dose (RPTD) was defined as the highest dose level at which only one or none of the six patients experienced a DLT. At the RPTD, additional four to a total of 10 patients would be treated.
Oxaliplatin pharmacokinetics
Oxaliplatin blood levels in dose levels 1 and 2 were determined on days 1–2 of cycle 1 (bortezomib administered prior to oxaliplatin) and on days 15–16 of cycle 1 (oxaliplatin administered prior to bortezomib). Blood was sampled at baseline (prior to bortezomib) and at 0.5, 1, 2, 4, and 24 h after the completion of the oxaliplatin infusion. On Day 1, the 1-h time point was taken immediately prior to oxaliplatin administration. At each time point, 7 ml of blood was drawn from patients and collected in a green top tube. Samples were processed by refrigerated centrifugation at 1,000g and then processed by Amicon ultrafiltration within 1 h, transferred to freezer vials and stored at −20 °C until analyzed. Samples were kept on ice throughout the procedure and processed within 1 h of sample collection.
Results
Patient accrual, dose levels, and dose-limiting toxicities
Thirty patients were enrolled between July 2003 and June 2006 of which 25 (11 men and 14 women) underwent NT assessments when completing the second 14-day cycle (Table 2). The primary malignancies of evaluable patients were stomach/GE junction (5); pancreas (4); liver/biliary (4); ovarian (4); melanoma (3); colon and appendix (3), and urachus, head and neck, Hodgkin’s (1 each). One patient at dose level one was never dosed, and three withdrew early with no toxicity or efficacy information. The median number of 4-week cycles administered was 2 (range 1–10). The patients’ median age was 56 years (range 35–74 years); Drug administration is listed in Table 3. The majority of patients discontinued therapy because of progressive disease (22 patients); 5 patients discontinued therapy because of adverse events (Gastrointestinal-2; NT-2; Thrombocytopenia-1). One patient died of a complication of malignancy while on study. One patient elected to discontinue study therapy without experiencing either DLT or disease progression; a patient on dose level 1 died prior to receiving any treatment—and has been excluded from the Tables. The only other significant treatment-related toxicities were gastrointestinal symptoms and fatigue. Six patients developed nausea and vomiting at dose level 3 or higher and were managed by anti-emetics. Diarrhea was observed in one patient at dose level 1. DLTs prompting expansion occurred at dose level 4 (Table 4).
Neurotoxicity
NT was not dose limiting after completing cycle 2 in any of the 25 assessable patients. After a median of 3.4 cycles (range cycle 2–9) in the first 5 dose levels, grade 3 and 4 NT events were observed in 6 patients (Table 5). Among those 6 patients, one had grade 3 sensory neuropathy and grade 4 ataxia after 4 cycles, one had grade 3 ataxia and neurogenic hypotension after 5 cycles, one had grade 3 ataxia after 2 cycles, and 3 had grade 3 sensory neuropathy after 3–9 cycles. Consequently, a sixth dose level (Bortezomib 1.0 mg/m2 and Oxaliplatin 85 mg/m2) opened to include and expand cohort; none of the five patients who received a median of 2 cycles (range 1–6) experienced grade 3 or 4 NT events.
Anti-tumor activity
Evidence of activity was seen with 4 partial responses (1 biliary, 1 ampulla of Vater, 1 gastric, 1 ovarian). The gastric cancer patient had received 1 prior regimen (cisplatin and irinotecan). She was then treated with 4 cycles of oxaliplatin and bortezomib with a partial response and then underwent resection of metastatic disease, followed by adjuvant 5-FU and radiation. Seven years after treatment, she continues follow-up without evidence of disease. The ovarian cancer patient treated at dose level 6 had received 5 prior regimens of which 3 were platinum based. The last platinum-free interval was 11 months, and she sustained a near-total reduction of liver lesions and peritoneal disease after only 4 cycles of the combination of oxaliplatin and bortezomib; however, she had progression of disease after her eighth cycle of treatment on the protocol. The biliary cancer patient was treated at another hospital with several regimens and initially had a partial response to therapy after 2 cycles of treatment on study. Unfortunately, he progressed after his forth cycle and went on to receive FOLFOX and then single-agent gemcitabine. The ampulla of Vater cancer patient underwent a Whipple procedure to resect his disease in 2000, followed by adjuvant concurrent 5-FU and radiation. He presented 4 years later with metastatic disease and was started on this trial. After 4 cycles, he had a partial response. He continued on protocol until progressing after 9 cycles of treatment. Six other patients had stable disease.
Oxaliplatin pharmacokinetics (PK)
Data were obtained on five patients at dose levels 1 and 2. Figure 1 provides the results of ultrafilterable (UF) and total platinum on day 1 (pre-bortezomib) and on day 15 (post-bortezomib). Total platinum levels reveal the expected cumulative platinum concentrations with the second dose relative to the first for all patients for whom PK data were obtained.
Discussion
As expected, NT was dose limiting in this trial, with several potential mechanisms to be considered. Oxaliplatin causes an axonal sensory neuropathy, but peripheral nerve biopsies also demonstrate decreased myelinization [18]. The clinical syndrome of oxaliplatin sensory NT differs from that of cisplatin in which it tends to be two phased: acute cold-induced neuropathy which may affect the larynx, and is short-lived, followed by chronic and cumulative sensory neuropathy which is mainly reversible over time. The clinical syndrome of bortezomib-associated NT occurs acutely (usually within days of drug administration) and is manifested by pain and/or numbness of the distal extremities (often previously affected by prior therapies). It is reversible within days to weeks, but can progress for a few weeks following the last treatment. In this study, the neuropathy was different than usually seen with oxaliplatin in being seen much sooner, and different qualitatively with more ataxia and gait disorder, as well as neurogenic hypotension, consistent with cerebellar or posterior column neurotoxicity.
In the current study, the RPTD was determined to be bortezomib 1.0 mg/m2 and oxaliplatin 85 mg/m2, on a D1 and 4 every 2 weeks of bortezomib and day 1, every 2 weeks of oxaliplatin. Another study in 13 unpretreated patients with advanced colorectal cancer concluded that doses above 1 mg/m2 of bortezomib led to severe NT when receiving this drug in combination with oxaliplatin, leucovorin (LV), and 5-fluorouracil. Bortezomib was administered on days 1, 8, and 15; oxaliplatin was given at the fixed dose of 85 mg/m2 on days 1 and 15.
In another small phase I trial, 13 patients with advanced solid tumors were treated with oxaliplatin 130 mg/m2 intravenously on day 1, capecitabine 750–900 mg/m2 twice daily orally for 14 days, and bortezomib 1.0, 1.3, or 1.6 mg/m2 intravenously on days 1 and 8 of 21 day cycles [19]. No dose limiting toxicities were noted at all bortezomib dose levels when administered with full-dose capecitabine and oxaliplatin. Based on the results from single-agent trials, 1.6 mg/m2 was the highest bortezomib dose administered in this more widely spaced schedule. Only one patient experienced grade 3 peripheral neuropathy in cycle 8.
In conclusion, bortezomib in combination with oxaliplatin can be administered as a safe regimen. We observed anti-tumor activity in this heavily pretreated population as manifested by partial responses in 4 patients (1 biliary, 1 ampulla of Vater, 1 gastric, 1 ovarian). The RPTD in this regimen of bortezomib at D1, 4, 15, and 18 and oxaliplatin on D1 and 15 every 4 weeks was determined to be 1.0 and 85 mg/m2, respectively. Peripheral neuropathy, diarrhea, and fatigue were noted to be clinically significant toxicities. We believe that the addition of proteasome inhibition to cytoxtoxics, such as oxaliplatin, can modulate activity. The current trial clearly shows evidence of anti-tumor activity beyond that reported for single-agent oxaliplatin (which is generally inactive by itself), particularly in gastrointestinal and ovarian cancers. This approach may be more feasible using carfilzomib, which is a non-neurotoxic proteasome inhibitor.
References
Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ (1999) Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res 59(11):2615–2622
Rajkumar SV, Richardson PG, Hideshima T, Anderson KC (2005) Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol 23(3):630–639. doi:10.1200/JCO.2005.11.030
O’Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, Straus D, Portlock C, Hamlin P, Choi E, Dumetrescu O, Esseltine D, Trehu E, Adams J, Schenkein D, Zelenetz AD (2005) Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol 23(4):676–684. doi:10.1200/JCO.2005.02.050
Hamilton AL, Eder JP, Pavlick AC, Clark JW, Liebes L, Garcia-Carbonero R, Chachoua A, Ryan DP, Soma V, Farrell K, Kinchla N, Boyden J, Yee H, Zeleniuch-Jacquotte A, Wright J, Elliott P, Adams J, Muggia FM (2005) Proteasome inhibition with bortezomib (PS-341): a phase I study with pharmacodynamic end points using a day 1 and day 4 schedule in a 14-day cycle. J Clin Oncol 23(25):6107–6116. doi:10.1200/JCO.2005.01.136
Aghajanian C, Blessing JA, Darcy KM, Reid G, DeGeest K, Rubin SC, Mannel RS, Rotmensch J, Schilder RJ, Riordan W (2009) A phase II evaluation of bortezomib in the treatment of recurrent platinum-sensitive ovarian or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol 115(2):215–220. doi:10.1016/j.ygyno.2009.07.023
Mackay H, Hedley D, Major P, Townsley C, Mackenzie M, Vincent M, Degendorfer P, Tsao MS, Nicklee T, Birle D, Wright J, Siu L, Moore M, Oza A (2005) A phase II trial with pharmacodynamic endpoints of the proteasome inhibitor bortezomib in patients with metastatic colorectal cancer. Clin Cancer Res 11(15):5526–5533. doi:10.1158/1078-0432.CCR-05-0081
Cusack JC Jr, Liu R, Baldwin AS Jr (2000) Inducible chemoresistance to 7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothe cin (CPT-11) in colorectal cancer cells and a xenograft model is overcome by inhibition of nuclear factor-kappaB activation. Cancer Res 60(9):2323–2330
Howell SB, Safaei R, Larson CA, Sailor MJ (2010) Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol 77(6):887–894. doi:10.1124/mol.109.063172
Aghajanian C, Dizon DS, Sabbatini P, Raizer JJ, Dupont J, Spriggs DR (2005) Phase I trial of bortezomib and carboplatin in recurrent ovarian or primary peritoneal cancer. J Clin Oncol 23(25):5943–5949. doi:10.1200/JCO.2005.16.006
Cersosimo RJ (2005) Oxaliplatin-associated neuropathy: a review. Ann Pharmacother 39(1):128–135. doi:10.1345/aph.1E319
Cassidy J, Misset JL (2002) Oxaliplatin-related side effects: characteristics and management. Semin Oncol 29(5 Suppl 15):11–20. doi:10.1053/sonc.2002.35524
Pasetto LM, D’Andrea MR, Rossi E, Monfardini S (2006) Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol 59(2):159–168. doi:10.1016/j.critrevonc.2006.01.001
Gamelin L, Boisdron-Celle M, Delva R, Guerin-Meyer V, Ifrah N, Morel A, Gamelin E (2004) Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res 10(12 Pt 1):4055–4061. doi:10.1158/1078-0432.CCR-03-0666
Muto O, Ando H, Ono T, Itagaki H, Kobayashi Y, Onuki M, Akashi T, Tanaka Y, Hanaoka T (2007) Reduction of oxaliplatin-related neurotoxicity by calcium and magnesium infusions. Gan to kagaku ryoho Cancer Chemother 34(4):579–581
Levi F, Misset JL, Brienza S, Adam R, Metzger G, Itzakhi M, Caussanel JP, Kunstlinger F, Lecouturier S, Descorps-Declere A et al (1992) A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer 69(4):893–900
Kautio AL, Haanpaa M, Kautiainen H, Leminen A, Kalso E, Saarto T (2011) Oxaliplatin scale and National Cancer Institute-Common Toxicity Criteria in the assessment of chemotherapy-induced peripheral neuropathy. Anticancer Res 31(10):3493–3496
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Screnci D, McKeage MJ (1999) Platinum neurotoxicity: clinical profiles, experimental models and neuroprotective approaches. J Inorg Biochem 77(1–2):105–110
Cohen SJ, Engstrom PF, Lewis NL, Langer CJ, McLaughlin S, Beard M, Weiner LM, Meropol NJ (2008) Phase I study of capecitabine and oxaliplatin in combination with the proteasome inhibitor bortezomib in patients with advanced solid tumors. Am J Clin Oncol 31(1):1–5. doi:10.1097/COC.0b013e31805c142f
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobrinsky, B., Joseph, S.O., Muggia, F. et al. A phase I and pharmacokinetic study of oxaliplatin and bortezomib: activity, but dose-limiting neurotoxicity. Cancer Chemother Pharmacol 72, 1073–1078 (2013). https://doi.org/10.1007/s00280-013-2295-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2295-6