Abstract
Purpose
The present study evaluated the predictive and prognostic impact of initial fluorine-18-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) in patients with locally advanced rectal cancer treated with neoadjuvant concurrent chemoradiotherapy (CCRT).
Methods
Eighty-one consecutive patients with locally advanced rectal cancer (cT3-T4 N−/N+) treated with neoadjuvant CCRT were enrolled. The FDG-PET/CT parameters, including the SUVmax, metabolic tumor volume (MTV, 50 % of SUVmax), and multiplication of the SUVmean and MTV (total lesion glycolysis, TLG), were analyzed in relation to the pathologic response and disease recurrence.
Results
Five patients (6.2 %) achieved a pathologic complete response (pCR) after CCRT followed by surgery. None of the FDG-PET/CT parameters was identified as a predictive factor for pCR. After a median follow-up period of 26.7 (range 10.9–63.3) months, 19 patients (23.5 %) presented a local and/or distant recurrence. In a multivariate analysis including the clinicopathologic parameters, the TLG of the primary tumor was associated with a worse disease-free survival after neoadjuvant CCRT (HR 20.035, 95 % CI 1.726–232.559; P = 00.017).
Conclusions
The TLG of the primary tumor in the initial FDG-PET/CT can be considered as a prognostic factor for patients with locally advanced rectal cancer treated with neoadjuvant CCRT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant concurrent chemoradiotherapy (CCRT) followed by an optimal surgical technique is considered the standard of care for patients with locally advanced adenocarcinoma of the middle/low rectum, as it provides the opportunity to downstage tumors, increases sphincter preservation, and decreases the risk of locoregional recurrence when compared with postoperative treatment, even though no improvement in overall survival has been observed [1, 2]. However, the tumor response to neoadjuvant CCRT varies considerably among patients, ranging from the complete disappearance of the tumor in about 15–20 % of cases to a lack of any pathological change or even tumor progression during the treatment. It is also well known that rectal cancer patients who achieve pCR after neoadjuvant CCRT have a lower local recurrence rate and improved overall survival when compared to patients with residual cancer cells [3, 4]. Thus, to facilitate a better individualized multidisciplinary therapeutic approach, including preoperative treatment, surgery, and adjuvant treatment, for each patient, additional predictive and prognostic markers for pCR and early relapse have been actively sought in clinical research.
Standard imaging modalities, such as endoscopic transrectal ultrasound (ERUS), computed tomography (CT), and pelvic magnetic resonance imaging (MRI), allow a valid morphological assessment of the tumor extent at the initial diagnosis, yet their accuracy for restaging after neoadjuvant CCRT is very low as regards predicting pCR [5, 6].
Fluorine-18-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT), which evaluates the tissue metabolic activity using the glucose metabolism, is useful not only for staging but also for assessing the tumor response to treatment. Several previous studies have observed that FDG-PET/CT could be an indicator of treatment response, and in a neoadjuvant setting, it could have a predictive and/or prognostic value for many tumors, such as esophagogastric cancer [7], non-small-cell lung cancer [8], and breast cancer [9]. For rectal cancer, Dencke et al. [10, 11] reported that FDG-PET was superior to CT and MRI in predicting the response to the preoperative multimodal treatment of locally advanced rectal cancer. Moreover, Calvo et al. [12] found FDG-PET to be useful in assessing the chemoradiation response of locally advanced rectal cancer and suggested that the initial SUVmax may be of prognostic value as regards the long-term patient outcome. However, since the sample size of these studies was very small (fewer than 30 patients), it is difficult to draw a conclusion on the prognostic value of FDG-PET in the case of rectal cancer.
The metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are the tumor metabolic activity measures determined by FDG-PET/CT [13], and these functional parameters could have clinical value as regards treatment evaluation and disease prognostication.
Accordingly, this study evaluated the predictive and prognostic impact of the initial FDG-PET/CT in patients with locally advanced rectal cancer treated with neoadjuvant CCRT.
Patients and methods
Patient characteristics
The study population included consecutive patients with clinical T3/4, N−/+ rectal cancer suitable for neoadjuvant CCRT observed between December 2005 and July 2010. The diagnosis and staging of the rectal cancer were assessed according to the WHO classifications [14, 15]. The initial imaging TNM status was obtained from pretreatment MRI imaging, while the M stage was obtained from structural imaging and FDG-PET/CT; any discordance was resolved by confirmation at surgery, a biopsy, or subsequent follow-up imaging. All the patients underwent FDG-PET/CT before CCRT. The pathologic analysis of the surgical specimens was performed by the local pathologist department according to the ypTNM system [16].
Chemoradiotherapy
The chemoradiotherapy consisted of radiotherapy, delivered at a total dose of 4,500 cGy in 25 daily fractions of 1.8 Gy in combination with a concurrent chemotherapy regimen of a 5-fluorouracil plus leucovorin bolus infusion (‘Mayo’ regimen). All the patients underwent rectal surgery (total mesorectal excision) with curative intent 6 weeks after the end of the neoadjuvant treatment. Adjuvant chemotherapy using the ‘Mayo’ regimen was also delivered for 4 months.
PET protocol and measurement of tumor volume
All the patients fasted for at least 6 h before the administration of F-18 FDG, and the blood glucose concentration was confirmed to be less than 150 mg/dL. Approximately 8.1 MBq of F-18 FDG per kg of body weight was injected intravenously, and the patients were advised to rest for an hour before the acquisition of the FDG-PET/CT image. The PET/CT scans were performed using a Reveal HiRez (Siemens-CTI, Knoxville, TN, USA, 6-slice CT) and Discovery STE (GE Healthcare, Milwaukee, WI, USA, 16-slice CT). A low-dose CT scan was initially obtained for attenuation correction, followed by the PET scan at 3 min per bed position. The PET data were reconstructed iteratively based on an ordered-subset expectation maximization algorithm using the low-dose CT datasets for the attenuation correction. A standardized uptake value (SUV) was measured for all primary rectal cancer lesions and presented as the SUVmax. The PET/CT images were interpreted by two experienced nuclear medicine physicians, and a final consensus reached for all the patients. Regions of interest (ROIs) were placed manually over all the rectal tumors in the attenuation-corrected images, and the SUVmax within the ROIs was recorded.
The maximum and average standardized uptake values (SUVmax and SUVmean) were quantitatively used to determine the FDG-PET activity. The measured variables included the MTV, SUVmax, and SUVmean in the pretreatment scans, and the threshold intensity value used in this study was a SUV of 50 %. After segmenting all the hypermetabolic tumor foci, the software calculated the MTV, defined as the total volume of the primary tumor in the body, along with the maximum and average SUV within the MTV. The TLG was also calculated by multiplying the SUVmean of the primary tumor by the MTV. The quartile value of the TLG was used in all the analyses (range 13.09–763.32, Q1 < 43, Q2 43–85, Q3 85–145, and Q4 > 145). This study only analyzed the pretreatment FDG-PET/CT and does not address any changes in the SUV parameters following CCRT.
Clinical follow-up
The postoperative program included follow-up visits every 3 months for the first 2 years, then every 6 months for the following 3 years, and once annually thereafter. At each visit, clinical examinations were performed and the serum level of the carcinoembryonic antigen (CEA) was monitored. Chest X-rays and abdominal computed tomography scans were obtained every 6 months, plus a full colonoscopy was performed 6 months after surgery and then once every 3–5 years. FDG-PET/CT was ordered selectively in the case of any abnormalities during the examination.
Statistical analyses
The chi-square test or t test was used to analyze the correlation between the FDG-PET and clinicopathologic parameters. The prognostic significance of the primary tumor SUVmax, SUVmean, MTV, and TLG relative to disease-free survival (DFS) or overall survival (OS) was also analyzed. DFS was calculated from the date of diagnosis to the date of any events, including all local, regional, or distant recurrences. Kaplan–Meier curves were used to calculate the DFS and OS values. A multivariate analysis was performed according to the Cox proportional hazards model with the backward elimination of factors found to be statistically significant in the univariate analyses. All the tests were two sided and performed at a 5 % level of significance using SPSS (version 15.0; SPSS Inc., Chicago, IL).
Results
Patient characteristics
Eighty-one consecutive patients with locally advanced rectal cancer were enrolled at Kyungpook National University Hospital (Daegu, Korea) between December 2005 and July 2010. The clinicopathologic characteristics of these 81 patients are shown in Table 1. All the patients were operated on 6 weeks after the end of their CCRT: Complete resection (R0) was achieved in 78 (96.3 %) patients, whereas positive margins (R1) were found in two (2.5 %) patients, and one patient showed liver metastasis (R2) at surgery. Low anterior resection was performed on 67 (86.4 %) patients, while the others received an abdominoperineal resection. Laparoscopy surgery was performed on 57 (70.4 %) patients. Seventy-two patients (88.9 %) received postsurgical adjuvant chemotherapy.
Post-CCRT pathologic evaluation and FDG-PET/CT
Table 1 shows the post-CCRT pathologic staging in the surgical specimen. Five patients (6.2 %) demonstrated a pathologic complete response (pCR, ypT0N0), and 48 patients (59.3 %) experienced ypN0. When compared with the clinical baseline stage, primary tumor downstaging was documented in 33 patients (40.7 %) and locoregional nodal involvement downstaging found in 57 patients (70.4 %). At the baseline, all the patients presented abnormal 18F-FDG avidity at the site of the primary tumor. The mean baseline SUVmax was 10 (range 4.1–30.0), the mean MTV (threshold 50 %) was 12 mL (range 2.35–15.64 mL), and the mean TLG was 85 (range 13.09–763.32). Among the clinicopathologic factors, the differences in the TLG were not all significant in this study (Table 2), and none of the investigated parameters, including the tumor volume parameters (SUVmax, SUVmean, MTV, and TLG), was shown to be an independent predictive factor for pCR (Table 3).
Disease recurrence and FDG-PET/CT
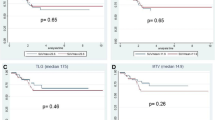
After a median follow-up of 26.7 (range 10.9–63.3) months, 19 patients (23.5 %) presented a recurrent disease (2 local, 15 distant, and 2 local + distant) and 12 (14.8 %) had died of a recurrent disease. Eighteen (94.7 %) of the patients with a recurrent disease had achieved less than pCR after CCRT. In the univariate analysis, the TLG of the primary tumor was found to be significantly associated with DFS and OS (Table 4). Moreover, the multivariate analysis demonstrated that the TLG of the primary tumor was an independent prognostic factor for DFS for locally advanced rectal cancer treated with neoadjuvant CCRT (HR 20.035, 95 % CI 1.726-232.559; P = 0.017), whereas the SUVmax and MTV were not significant prognostic factors (Table 5, Fig. 1).
Discussion
The current study found that the pretreatment TLG, a volumetric parameter of FDG-PET/CT, was an important prognostic factor for DFS in patients with locally advanced rectal cancer treated with neoadjuvant CCRT.
The use of FDG-PET/CT for staging and predicting tumor response is now one of the most rapidly expanding areas in diagnostic imaging. Rectal cancer is a disease model of particular interest, because an accurate and noninvasive method for evaluating the response to preoperative CCRT could lead to patient selection for minimally invasive surgical approaches or even the selection of candidates for additional chemotherapy [17, 18]. A systematic review of monitoring and predicting the response to therapy using FDG-PET/CT in the case of rectal cancer was recently carried out by de Geus-Oei et al. [19]. They identified and analyzed a series of 19 studies, although almost all the studies were very small and heterogeneous as regards the methods applied for PET quantification (visual FDG-PET response, SUVmax, SUVmean, and TLG), the timing of the examination, metabolic response evaluation criteria, and clinical end points. Yet, despite such strong limitations for drawing consistent conclusions, the authors consider that most of the studies showed that FDG-PET was ‘a significant predictor of therapy outcome’ [19]. Notwithstanding, in the present study, none of the investigated parameters, including the SUVmax, SUVmean, MTV, and TLG, was identified as an independent predictive factor for pCR, even though the patients who achieved pCR had a lower initial SUV value than the non-pCR patients. Possible explanations for these results are that the sample size of the current study was relatively small and only 6.2 % of the patients achieved pCR after neoadjuvant CCRT due to the relatively low dose of radiation (4500 cGy) and intermittent infusion of 5-fluorouracil in the current study. Martoni et al. [20] also reported that while the baseline PET expressed as SUV-1 was correlated with the pathologic response, this correlation was lost in a multivariate analysis. Thus, they concluded that FDG-PET/CT as a baseline did not appear to have any relevance in the standard staging workup as a predictor of the pathologic response.
The use of a semiquantitative index for the tumor FDG uptake, such as the SUV, is one possible source of metabolic information, and several studies have already shown that the SUVmax of the primary tumor is useful for determining the prognosis in patients with rectal cancer [12, 20]. However, in the current study, the SUVmax of the primary tumor was not identified as a significant prognostic factor for DFS or OS. While the SUVmax is a robust and convenient quantitative measure, it has been argued that since it is only the measurement of a single pixel with the highest radiotracer concentration within the ROI, it may not reflect the heterogeneous nature of the tumor. Thus, the TLG has been proposed as a more accurate parameter, which takes account of both the metabolic activity (SUVmean or SUVmax) and the tumor volume [21]. In the present study, the DFS for the patients with a high TLG for the primary tumor was worse than that for the patients with a low TLG (P = 0.017). These findings are consistent with the prior study by Gulec et al. [22], who found a statistically significant association between the functional tumor parameters (TLG) and the clinical outcomes in patients with colorectal cancer with liver metastasis. In their study, the median survival for patients with pretreatment TLG values above and below 600 g was 11.2 and 26.9 months, respectively (P < 0.05). Thus, they concluded that the pretreatment TLG could be a useful predictive marker for survival. Recent studies have also reported similar TLG-related outcomes with other solid tumors, including malignant pleural mesothelioma [23], non-small-cell lung cancer [24], and nasopharyngeal carcinoma [25, 26]. The prognostic benefits of pCR have already been proven in patients with locally advanced rectal cancer treated with neoadjuvant CCRT. Plus, since PET can illustrate changes in the tumor biology and provide an earlier assessment of the cancer response following CCRT, sequential PET has been shown to provide a more accurate prediction of the pathological response of locally advanced rectal cancer than other anatomic staging systems. Several studies have also showed that, irrespective of the pretreatment T stage, a poorer metabolic response obtained by FDG-PET/CT can be an independent predictor of recurrence [20, 27, 28]. Accordingly, the metabolic response assessed by FDG-PET/CT may be an alternative approach of pathologic staging to stratify the management of patients with locally advanced rectal cancer.
In conclusion, the TLG of the primary tumor in the initial FDG-PET/CT can be considered as a prognostic factor for neoadjuvant CCRT in patients with rectal cancer. As such, this value can be a useful quantitative criterion for patient selection and disease prognostication, leading to more appropriate and efficient clinical treatment with improved long-term outcomes. Additional prospective studies with larger numbers of patients are needed to validate the prognostic utility of this promising functional biomarker derived from FDG-PET/CT.
References
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Pucciarelli S, Toppan P, Friso ML, Russo V, Pasetto L, Urso E, Marino F, Ambrosi A, Lise M (2004) Complete pathologic response following preoperative chemoradiation therapy for middle to lower rectal cancer is not a prognostic factor for a better outcome. Dis Colon Rectum 47:1798–1807
Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23:8688–8696
Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, Cellini F, Barbaro B, Cogliandolo S, Nuzzo G, Doglietto GB, Ambesi-Impiombato F, Cosimelli M (2002) Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys 53:664–674
Rau B, Hunerbein M, Barth C, Wust P, Haensch W, Riess H, Felix R, Schlag PM (1999) Accuracy of endorectal ultrasound after preoperative radiochemotherapy in locally advanced rectal cancer. Surg Endosc 13:980–984
Chen CC, Lee RC, Lin JK, Wang LW, Yang SH (2005) How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum 48:722–728
Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, Meisetschlager G, Busch R, Siewert JR, Schwaiger M, Fink U (2001) Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 19:3058–3065
Mac Manus MP, Hicks RJ, Matthews JP, McKenzie A, Rischin D, Salminen EK, Ball DL (2003) Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol 21:1285–1292
Martoni AA, Zamagni C, Quercia S, Rosati M, Cacciari N, Bernardi A, Musto A, Fanti S, Santini D, Taffurelli M (2010) Early (18)F-2-fluoro-2-deoxy-d-glucose positron emission tomography may identify a subset of patients with estrogen receptor-positive breast cancer who will not respond optimally to preoperative chemotherapy. Cancer 116:805–813
Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Gutberlet M, Hunerbein M, Felix R, Wust P, Amthauer H (2005) Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol 15:1658–1666
Amthauer H, Denecke T, Rau B, Hildebrandt B, Hunerbein M, Ruf J, Schneider U, Gutberlet M, Schlag PM, Felix R, Wust P (2004) Response prediction by FDG-PET after neoadjuvant radiochemotherapy and combined regional hyperthermia of rectal cancer: correlation with endorectal ultrasound and histopathology. Eur J Nucl Med Mol Imaging 31:811–819
Calvo FA, Domper M, Matute R, Martinez-Lazaro R, Arranz JA, Desco M, Alvarez E, Carreras JL (2004) 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys 58:528–535
Erdi YE, Macapinlac H, Rosenzweig KE, Humm JL, Larson SM, Erdi AK, Yorke ED (2000) Use of PET to monitor the response of lung cancer to radiation treatment. Eur J Nucl Med 27:861–866
Hamilton SRAL (2000) WHO classification. IARC Press, Lyon
Greene FLPD, Fleming ID (2002) The AJCC cancer staging manual, 6th edn. Springer, New York
Brierley JD, Greene FL, Sobin LH, Wittekind C (2006) The “y” symbol: an important classification tool for neoadjuvant cancer treatment. Cancer 106:2526–2527
Habr-Gama A, Perez RO (2009) Non-operative management of rectal cancer after neoadjuvant chemoradiation. Br J Surg 96:125–127
Neuman HB, Elkin EB, Guillem JG, Paty PB, Weiser MR, Wong WD, Temple LK (2009) Treatment for patients with rectal cancer and a clinical complete response to neoadjuvant therapy: a decision analysis. Dis Colon Rectum 52:863–871
de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ (2009) Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med 50(Suppl 1):43S–54S
Martoni AA, Di Fabio F, Pinto C, Castellucci P, Pini S, Ceccarelli C, Cuicchi D, Iacopino B, Di Tullio P, Giaquinta S, Tardio L, Lombardi R, Fanti S, Cola B (2011) Prospective study on the FDG-PET/CT predictive and prognostic values in patients treated with neoadjuvant chemoradiation therapy and radical surgery for locally advanced rectal cancer. Ann Oncol 22:650–656
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, Casilla C, Fazzari M, Srivastava N, Yeung HW, Humm JL, Guillem J, Downey R, Karpeh M, Cohen AE, Ginsberg R (1999) Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using pet-fdg imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 2:159–171
Gulec SA, Suthar RR, Barot TC, Pennington K (2011) The prognostic value of functional tumor volume and total lesion glycolysis in patients with colorectal cancer liver metastases undergoing 90Y selective internal radiation therapy plus chemotherapy. Eur J Nucl Med Mol Imaging 38:1289–1295
Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, Ahn MJ, Kim TS, Yi CA, Chung MJ (2010) Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol 17:2787–2794
Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, Shih MC, Shimada N, Chen S, Salgia R, Appelbaum DE, Suzuki K, Chen CT, Pu Y (2011) Prognostic value of metabolic tumor burden on (18)F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging
Chan WK, Mak HK, Huang B, Yeung DW, Kwong DL, Khong PL (2010) Nasopharyngeal carcinoma: relationship between 18F-FDG PET-CT maximum standardized uptake value, Metabolic tumour volume and total lesion glycolysis and TNM classification. Nucl Med Commun 31:206–210
Chan SC, Chang JT, Lin CY, Ng SH, Wang HM, Liao CT, Chang CJ, Lin SY, Yen TC (2011) Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun 32:989–996
Leibold T, Akhurst TJ, Chessin DB, Yeung HW, Macapinlac H, Shia J, Minsky BD, Saltz LB, Riedel E, Mazumdar M, Paty PB, Weiser MR, Wong WD, Larson SM, Guillem JG (2011) Evaluation of 18F-FDG-PET for early detection of suboptimal response of rectal cancer to preoperative chemoradiotherapy: a prospective analysis. Ann Surg Oncol 18:2783–2789
Yeung JM, Kalff V, Hicks RJ, Drummond E, Link E, Taouk Y, Michael M, Ngan S, Lynch AC, Heriot AG (2011) Metabolic response of rectal cancer assessed by 18-FDG PET following chemoradiotherapy is prognostic for patient outcome. Dis Colon Rectum 54:518–525
Acknowledgments
This study was supported by a grant from the Korea Health Technology R and D Project, Ministry of Health and Welfare, Republic of Korea (A111345).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Gyu Seog Choi and Jong Gwang Kim contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lee, S.J., Kim, J.G., Lee, SW. et al. Clinical implications of initial FDG-PET/CT in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Cancer Chemother Pharmacol 71, 1201–1207 (2013). https://doi.org/10.1007/s00280-013-2114-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2114-0