Abstract
Purpose
To evaluate the efficacy and safety of weekly paclitaxel in patients with recurrent or metastatic head and neck cancer (HNC) by combined analysis of early and late phase II trials.
Methods
Eligibility criteria included histologically proven HNC with recurrent or metastatic disease, measurable disease, PS 0-2, and one or no prior chemotherapy regimens. Treatment consisted of a 1-h infusion of paclitaxel at a dose of 100 mg/m2 weekly for 6 weeks of a 7-week cycle. A total of 74 patients were enrolled: 37 between February and November 2004 in an early phase II trial and 37 between October 2005 and July 2006 in a late phase II trial.
Results
The median number of treatment cycles was two, and median dose intensity was 84.2 mg/m2/week. The most common grade 3–4 adverse events were leukopenia (37.5%), neutropenia (30.6%), anemia (12.5%), constipation (8.3%), peripheral neuropathy (5.6%), anorexia (5.6%), and pneumonitis (5.6%). Overall response rate was 29.0% according to RECIST. The median duration of response, median time to progression, and median survival time were 7.4, 3.4, and 14.3 months, respectively.
Conclusions
This study demonstrates that weekly paclitaxel has promising activity with acceptable toxicity in the treatment of recurrent or metastatic HNC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and necks cancers (HNCs) are the sixth most common cancers worldwide, and approximately 500,000 new cases are projected annually [22]. An estimated 60% of these patients present with locally advanced disease (stage III/IV) [32]. Although the treatment of these locally advanced HNC has progressed, half will recur. While some of these are suitable for salvage treatment, including surgery or chemoradiotherapy, most are scheduled to receive palliative chemotherapy only.
Platinum-based combination chemotherapy is widely used as first-line treatment for recurrent/metastatic HNC. However, while several randomized trials have suggested that combination chemotherapy yields superior response rates, it is also associated with increased toxicity and no significant survival advantage over single agent chemotherapy [1, 4, 5, 15, 31, 35]. A recent randomized trial of platinum-based chemotherapy with or without cetuximab demonstrated significant survival benefit in the arm receiving cetuximab [30]. However, cetuximab was not given to patients in the control arm at the time of progression and it therefore remains unanswered whether the addition of cetuximab to first-line chemotherapy provides a survival benefit over sequential use of platinum-based chemotherapy followed by cetuximab at the time of progression. In other words, standard therapy in first-line treatment for recurrent/metastatic HNC has not yet been established. Furthermore, treatment options for patients who are refractory to platinum-based chemotherapy are limited. Optimal treatment options for these patients are therefore desirable.
Paclitaxel is a novel diterpenoid isolated from the bark of the Pacific yew, Taxus brevifolia [34]. Paclitaxel has high-affinity binding to microtubules, promotes microtubule assembly, and stabilizes tubulin polymers against depolymerization affecting cells in the G2/M-phase [24, 26].
Previous studies of high-dose tri-weekly paclitaxel (200–250 mg/m2) in patients with advanced or recurrent/metastatic HNC demonstrated treatment activity, with an overall response of 35–40%, but that this regimen was associated with severe neuropathy and myelosuppression [6, 27]. Since the survival of patients with recurrent or metastatic HNC is limited, additional consideration should be given to their quality of life.
Previous studies of weekly paclitaxel at a reduced single dose for other cancers demonstrated comparable efficacy to a high-dose tri-weekly regimen with milder toxicities, including neuropathy and myelosuppression [28].
At the time the present trials were planned, only one prospective phase II study of weekly paclitaxel in the treatment of recurrent or metastatic HNC had appeared. Results showed acceptable toxicities but the poor response rate of 9.3% (4/43) [3]. Thus, no data were available to support the practical use of weekly paclitaxel in the treatment of recurrent or metastatic HNC, albeit that weekly paclitaxel has been widely used in the treatment of HNC patients who are refractory to a platinum-based chemotherapy.
Here, therefore, we conducted two multicenter, phase II trials, an early and late phase II trial of weekly paclitaxel in patients with recurrent or metastatic HNC, to evaluate efficacy and safety in the two trials and to confirm data on safety and efficacy between them.
Patients and methods
The subjects of the present study were patients enrolled in two multicenter trials, an early and a late phase II trial of weekly paclitaxel in the treatment of recurrent or metastatic HNC. To allow the safety and efficacy of these trials to be compared, they were conducted under the same design. Each trial was conducted at 19 institutions in Japan.
Eligibility criteria included histologically or cytologically proven HNC with recurrent or metastatic disease; age 20 years or older but less than 75; a measurable lesion; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2; adequate organ function, as defined by an absolute neutrophil count (ANC) >2,000/μL, platelet count >100,000/μL, hemoglobin >9.0 g/dL, AST <100 IU/L, ALT <100 IU/L, total bilirubin <1.5 mg/dL, and serum creatinine <1.5 mg/dL; and life expectancy >2 months from the beginning of treatment. Patients were excluded if they had received two or more prior regimens of chemotherapy for recurrent/metastatic HNC. The study protocol was reviewed and approved by the ethics committee of each of the participating institutions before patient enrollment began. Informed consent was obtained from all patients.
Treatment
On the basis of the results of a phase I trial of weekly paclitaxel in solid tumors [20], patients in both the early and late phase trials received a 1-h iv infusion of paclitaxel at a dose of 100 mg/m2 weekly over a 7-week cycle on days 1, 8, 15, 22, 29, and 36, followed by 2 weeks of rest until unacceptable toxicity, patient refusal, or disease progression were observed. Patients received premedication with 8 mg dexamethasone (iv), 50 mg ranitidine (iv), and 50 mg diphenhydramine hydrochloride (po) 30–60 min prior to paclitaxel infusion.
Dose modification of paclitaxel by 20 mg/m2 was allowed if a patient experienced any of the following adverse events: (1) febrile neutropenia, (2) grade 3 or 4 thrombocytopenia, (3) grade 3 or 4 non-hematological toxicity, (4) grade 2 or higher peripheral neuropathy or myalgia/arthralgia, or (5) any toxicity that caused a dose to be skipped or required a dose reduction at the discretion of the physician. Dose reduction to less than 60 mg/m2 was not allowed.
Study endpoints
The primary endpoints in each trial were safety and response rate as assessed by WHO criteria, which could be compared to historical data. Secondary endpoints were duration of response, response rate based on the response evaluation criteria in solid tumors (RECIST), median time to progression (TTP), and median survival time (MST). The response rates and adverse events were evaluated by an independent safety and efficacy assessment committee. Responses were assessed by CT and/or MRI scans every 4 weeks. Adverse events were evaluated every week according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE) version 2.0. A subject’s TTP was defined as the time from the date of the enrollment in the present study to the first documentation of disease progression, subsequent therapy, or death. The duration of response was defined as the time from the date of the first confirmation of response to the first documentation of disease progression.
Statistical design
To confirm safety and efficacy, applications for approval of anti-neoplastic drugs in Japan typically require two studies conducted under the identical design, an early and a late phase II trial. If the early trial does not demonstrate promising activity, the late trial is withheld. In each of the present studies, the expected response rate was considered to be 25% and the threshold response rate was set at 10%. Thirty-six patients were needed to evaluate efficacy in each study in order to reject the hypothesis that the true efficacy rate was below the threshold response rate, giving α = 0.025 (one-sided) and β = 0.3. A survival curve was estimated using the Kaplan–Meier method [16]. In the present trials, safety and efficacy analyses were conducted on an intention-to-treat (ITT) population, defined as all patients enrolled in the study who received at least one dose of paclitaxel. All statistical analyses were carried out using SAS Version 8.2.
Results
Patient characteristics
A total of 74 patients were enrolled, 37 between February and November 2004 in the early phase II trial and 37 between October 2005 and July 2006 in the late phase II trial. The two trials had one patient each who did not receive any administration of paclitaxel due to PS 3 or ANC <2,000/μL. Patient characteristics are shown in Table 1. Of note, a total of 25 (34.7%) patients had advanced cancer, 47 (65.3%) had recurrent cancer, and 62 (86.1%) had a prior history of chemotherapy, including platinum-based chemotherapy (76.4%). Of these, 23 (31%) had received prior platinum-based chemotherapy for recurrent/metastatic disease. No relevant differences in patient characteristics were observed between individuals in the early and late phase trial groups.
Treatment administration
For both the early and late phase trials, the combined median number of treatment cycles was 2.0 (range 1–10) and the median number of doses was 12 (range 1–50). The combined median interval between cycles was 14.0 days (range 13–28 days), and the median dose intensity was 84.2 mg/m2/week (range 43.0–107.7 mg/m2/week).
Safety
The safety evaluation was conducted in 72 patients who received at least one dose of paclitaxel. Adverse events are shown in Table 2. The most common grade 3–4 non-hematological adverse events were constipation (8.3%), peripheral neuropathy (5.6%), anorexia (5.6%), and pneumonitis (5.6%), while grade 3–4 hematological adverse events were leukopenia (37.5%), neutropenia (30.6%), and anemia (12.5%). No deaths related to paclitaxel treatment were seen during the study period. The incidence of greater than grade 2 peripheral neuropathy was 25.0% (18/72).
The percentage of patients requiring dose reductions was 34.7% (25/72). Although 16.7% (12/72) of patients required cessation of therapy, only 5.6% (4/72) was unable to complete the protocol of at least one cycle of paclitaxel. The most common reason for cessation was peripheral neuropathy, seen in 6.9% (5/72) of patients. The median time to onset of peripheral neuropathy was 34 days (range 1–141), and the median dose of onset was 500 mg/m2 (range 100–1600 mg/m2). In those patients who experienced peripheral neuropathy, 14.5% (8/55) recovered, 7.3% (4/55) remitted, and 78.2% (43/55) failed to recover by the end of the protocol.
Efficacy
Thirty-six patients in each study were assessed for efficacy (Table 3). Overall response rates (RRs) in the early and late trial were 33.3% (95% CI: 18.6, 51.0%) and 36.1% (95% CI: 20.8, 53.8%), respectively. In combined analysis of two trials, RR according to WHO and RECIST criteria were 34.7% (95% CI: 23.9, 46.9%) and 29.0% (95% CI: 18.7, 41.2%), respectively. RR according to the WHO criteria in the 55 patients who received prior platinum-based chemotherapy was 32.7% and 30.4% in the 23 patients who received prior platinum-based chemotherapy for recurrent/metastatic disease (Table 4). RR in the 60 patients who received prior radiotherapy, including adjuvant therapy, neoadjuvant therapy, and chemoradiotherapy, was 30.0 and 58.3% in the 12 patients who did not receive prior radiotherapy.
The median duration of response was 8.5 months (95% CI: 5.4, 11.5 months) in the early trial, 6.9 months (95% CI: 3.2, 7.9 months) in the late trial, and 7.4 months (95% CI: 5.4, 9.4 months) in total.
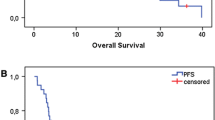
The median follow-up period in all patients was 13.8 months (range: 1.6-33.8 months). Median TTP and MST were 3.4 months (95% CI: 3.0, 4.6 months; Fig. 1) and 14.3 months (95% CI: 11.0, 19.4 months; Fig. 2), respectively. In the 64 patients excluding those with nasopharyngeal cancer, median TTP and MST were 3.2 months (95% CI: 2.9, 4.3 months) and 13.0 months (95% CI: 9.9, 16.9 months), respectively. As 11 patients (15.3%) had non-squamous cell carcinomas histology, which included 4 with adenoid cystic carcinoma and 7 with either mucoepidermoid tumor, adenocarcinoma, poorly differentiated carcinoma, acinar cell carcinoma, carcinoma, large cell carcinoma, or undifferentiated carcinoma, MST was also determined excluding these patients. MST was 13.4 months in the 61 patients with squamous cell carcinomas and 11.7 months in the 45 patients with squamous cell carcinomas of the oral cavity, paranasal cavity, oropharynx, hypopharynx, and larynx cancer. In the 23 patients who had received prior platinum-based chemotherapy for recurrent/metastatic disease, median TTP and MST were 3.2 months (95% CI: 2.5, 6.7 months) and 11.4 months (95% CI: 7.4, 19.4 months), respectively.
Discussion
Here, we conducted early and late phase II trials of weekly paclitaxel in patients with recurrent or metastatic HNC. Results demonstrated comparable safety and efficacy between the two trials. Further, the combined RR of the two trials was comparable to those previously reported in studies of tri-weekly paclitaxel in patients with advanced or recurrent HNC [6, 27]. All adverse events that occurred in the two trials were manageable, and no treatment-related deaths were observed. Although most patients had received prior chemotherapy, MST was 14.3 months, which was superior to that of previous studies in first-line patients with recurrent or metastatic HNC.
Of interest, MST in the 64 patients excluding those with nasopharyngeal cancer and in the 23 who had received prior platinum-based chemotherapy for recurrent/metastatic disease was 13.0 and 11.4 months, respectively. Allowing for the fact that this was a nonrandomized trial with a relatively small number of patients, these results are nevertheless better than those in the previous studies, particularly in showing that weekly paclitaxel was active in the treatment of HNC whether patients had received prior platinum-based chemotherapy or not.
Recently, the addition of cetuximab to platinum-based chemotherapy was shown to significantly prolong overall survival without exacerbating chemotherapy-associated toxicity or quality of life in patients with recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN) [10]. Furthermore, the addition of cetuximab to paclitaxel was also shown to exert promising activity in a first-line setting of a phase II trial, which had an RR of 71% and a complete response rate of 20%. Weekly paclitaxel might therefore be a good alternative to platinum-based chemotherapy for first-line patients with recurrent or metastatic SCCHN.
Treatment options for patients with recurrent or metastatic HNC who are refractory to platinum-based chemotherapy are limited. Several second-line chemotherapy regimens with cytotoxic agents, including methotrexate, vinorelbine, bleomycin, docetaxel, and S-1, have been investigated in the treatment of patients with recurrent or metastatic HNC after previous platinum-based chemotherapy [7, 11–14, 36]. Response rates and MST in these studies were 10–46.2% and less than 5 months, respectively, and it has accordingly not been possible to draw definitive conclusions on their clinical benefit.
Recently, a single institutional prospective study of weekly paclitaxel (80 mg/m2, weekly, 6 consecutive weeks) in SCCHN patients in whom platinum-based chemotherapy failed demonstrated a response rate of 43.3% and MST of 8.5 months [9]. Although this rate is superior to that of the present study, the study was conducted at a single institution and had no independent safety and efficacy assessment committee, while our study was a multicenter trial with independent safety and efficacy assessment committees. Further, our present study demonstrated a better duration of response and survival, which might be associated with the higher dose of paclitaxel in the present study.
A combined analysis of second-line use of cetuximab with or without platinum-based chemotherapy for patients with recurrent/metastatic SCCHN in whom platinum-based chemotherapy failed concluded that cetuximab would be effective as monotherapy and could be considered a therapeutic option [29]. However, the response rate, median TTP and MST of cetuximab alone in these patients were 13%, 2.3, and 5.9 months, respectively, indicating the need for further optimization of treatment options.
Although the number of patients who had previously received platinum-based chemotherapy for recurrent/metastatic disease in the present study was small, weekly paclitaxel showed a superior response rate and survival to that of previously reported agents and may therefore also be promising in second-line treatment following cisplatin-based regimens. Recently, weekly taxane-based chemotherapy was shown to exhibit promising activity as an induction chemotherapy in the primary therapy setting [17, 25, 33], suggesting that this dose-dense strategy may be particularly applicable to sequential treatment programs for HNC.
Long-term administration of weekly paclitaxel increases the incidence and severity of peripheral neuropathy, which often reduces quality of life. In our present patients who experienced peripheral neuropathy, 14.5% recovered and 7.3% remitted, while 78.2% failed to recover by the end of the protocol. Such sustained peripheral neuropathy may be limiting for patients receiving longer-term palliative therapy. Several studies have investigated anti-neuropathy drugs, including amifostine, gabapentin, and vitamin E, but all failed to demonstrate any benefit for these patients [2, 8, 18, 19, 21, 23]. The development of effective anti-neuropathy drugs is desirable.
Several limitations of the present study warrant mention. First, subjects included eight patients with nasopharyngeal cancer, which is considered to carry a better prognosis than other HNCs. Second, subjects included chemo-naive patients and patients who had not been confirmed to be refractory to platinum-based chemotherapy. Third, the present trials were nonrandomized, and differences in patient populations due to selection bias may have influenced outcomes and toxicity rates and thereby limit comparisons between studies. Fourth, the study included a range of histological subtypes. In other words, the subjects represented a markedly heterogeneous population.
In summary, this study demonstrated that weekly paclitaxel has promising activity with acceptable toxicity in the treatment of recurrent or metastatic HNC. Paclitaxel may be a good treatment option for recurrent or metastatic HNC.
References
The Liverpool Head and Neck Oncology Group (1990) A phase III randomised trial of cisplatinum, methotrextate, cisplatinum + methotrexate and cisplatinum + 5-FU in end stage squamous carcinoma of the head and neck. Br J Cancer 61:311–315
Argyriou AA, Chroni E, Koutras A, Ellul J, Papapetropoulos S, Katsoulas G, Iconomou G, Kalofonos HP (2005) Vitamin E for prophylaxis against chemotherapy-induced neuropathy: a randomized controlled trial. Neurology 64:26–31
Brotherton T (2001) Multicenter phase II trial of paclitaxel (taxol) (T): weekly one-hour infusion in previously treated advanced head/neck cancer (AHNC). ASCO, p 231a (abstract 921)
Clavel M, Vermorken JB, Cognetti F, Cappelaere P, de Mulder PH, Schornagel JH, Tueni EA, Verweij J, Wildiers J, Clerico M et al (1994) Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC Head and Neck Cancer Cooperative Group. Ann Oncol 5:521–526
Forastiere AA, Metch B, Schuller DE, Ensley JF, Hutchins LF, Triozzi P, Kish JA, McClure S, VonFeldt E, Williamson SK et al (1992) Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck: a Southwest Oncology Group study. J Clin Oncol 10:1245–1251
Forastiere AA, Shank D, Neuberg D, SGt Taylor, DeConti RC, Adams G (1998) Final report of a phase II evaluation of paclitaxel in patients with advanced squamous cell carcinoma of the head and neck: an Eastern Cooperative Oncology Group trial (PA390). Cancer 82:2270–2274
Gebbia V, Valenza R, Testa A, Borsellino N, Cannata G, Restivo S, Speciale R, Ingria F, Spadafora G, Gebbia N (1994) Methotrexate, vinblastine, epidoxorubicin, and bleomycin as second-line chemotherapy for recurrent and/or metastatic squamous cell carcinoma of the head and neck. ORL J Otorhinolaryngol Relat Spec 56:279–282
Gelmon K, Eisenhauer E, Bryce C, Tolcher A, Mayer L, Tomlinson E, Zee B, Blackstein M, Tomiak E, Yau J, Batist G, Fisher B, Iglesias J (1999) Randomized phase II study of high-dose paclitaxel with or without amifostine in patients with metastatic breast cancer. J Clin Oncol 17:3038–3047
Grau JJ, Caballero M, Verger E, Monzo M, Blanch JL (2009) Weekly paclitaxel for platin-resistant stage IV head and neck cancer patients. Acta Otolaryngol 129:1294–1299
Herrero FR, Hitt R, Kawecki A, Rottey S, Peyrade F, Vynnychenko I, Curran D, Kisker O, Gross A, Vermorken JB (2008) Cetuximab plus platinum-based therapy first line in patients with recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): A quality of life (QOL) analysis of the EXTREME trial. 33rd ESMO Congress. Annals of Oncology, Stockholm, Sweden, p 219 (abstract 693PD)
Inuyama Y, Kataura A, Togawa K, Saijo S, Satake B, Takeoda S, Konno A, Ebihara S, Sasaki Y, Kida A, Kanzaki J, Ichikawa G, Kono N, Moriyama H, Kamata S, Miyake H, Sakai M, Horiuchi M, Kubota A, Tsukuda M, Matsuura H, Baba S, Saito H, Matsunaga T, Taguchi T et al (1999) Late phase II clinical study of RP56976 (docetaxel) in patients with advanced/recurrent head and neck cancer. Gan To Kagaku Ryoho 26:107–116
Inuyama Y, Kida A, Tsukuda M, Kohno N, Satake B (1998) Early phase II study of S-1 in patients with advanced head and neck cancer. S-1 Cooperative Study Group (Head and Neck Working Group). Gan To Kagaku Ryoho 25:1151–1158
Inuyama Y, Kida A, Tsukuda M, Kohno N, Satake B (2001) Late phase II study of S-1 in patients with advanced head and neck cancer. Gan To Kagaku Ryoho 28:1381–1390
Iop A, Cartei G, Isaia A (1998) Vinorelbine, bleomycin and methotrexate as a salvage therapy for patients with head and neck squamous carcinoma in relapse after cisplatin/fluorouracil. Ann Oncol 9:225–227
Jacobs C, Lyman G, Velez-Garcia E, Sridhar KS, Knight W, Hochster H, Goodnough LT, Mortimer JE, Einhorn LH, Schacter L et al (1992) A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol 10:257–263
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Kies MS, Holsinger FC, Lee JJ, William WN Jr, Glisson BS, Lin HY, Lewin JS, Ginsberg LE, Gillaspy KA, Massarelli E, Byers L, Lippman SM, Hong WK, El-Naggar AK, Garden AS, Papadimitrakopoulou V (2010) Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol 28:8–14
Kottschade LA, Sloan JA, Mazurczak MA, Johnson DB, Murphy B, Rowland KM, Smith DA, Berg A, Stella PJ, Loprinzi CL (2009) The use of vitamin E for prevention of chemotherapy-induced peripheral neuropathy: a phase III double-blind, placebo controlled study-N05C3. 2009 ASCO Annual Meeting. J Clin Oncol (Orlando), p 491s (abstract 9532)
Leong SS, Tan EH, Fong KW, Wilder-Smith E, Ong YK, Tai BC, Chew L, Lim SH, Wee J, Lee KM, Foo KF, Ang P, Ang PT (2003) Randomized double-blind trial of combined modality treatment with or without amifostine in unresectable stage III non-small-cell lung cancer. J Clin Oncol 21:1767–1774
Nokihara H, Tamura T, Matsumoto Y (2002) Weekly paclitaxel in solid tumor, a phase I trial. Jpn J Lung Cancer. In: 43rd Annual meeting, abstract 395
Openshaw H, Beamon K, Synold TW, Longmate J, Slatkin NE, Doroshow JH, Forman S, Margolin K, Morgan R, Shibata S, Somlo G (2004) Neurophysiological study of peripheral neuropathy after high-dose Paclitaxel: lack of neuroprotective effect of amifostine. Clin Cancer Res 10:461–467
Parkin DM, Pisani P, Ferlay J (1999) Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer 80:827–841
Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY (2007) Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 110:2110–2118
Rowinsky EK, Donehower RC, Jones RJ, Tucker RW (1988) Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res 48:4093–4100
Salama JK, Stenson KM, Kistner EO, Mittal BB, Argiris A, Witt ME, Rosen F, Brockstein BE, Cohen EE, Haraf DJ, Vokes EE (2008) Induction chemotherapy and concurrent chemoradiotherapy for locoregionally advanced head and neck cancer: a multi-institutional phase II trial investigating three radiotherapy dose levels. Ann Oncol 19:1787–1794
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667
Smith RE, Thornton DE, Allen J (1995) A phase II trial of paclitaxel in squamous cell carcinoma of the head and neck with correlative laboratory studies. Semin Oncol 22:41–46
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J (2008) Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer 112:2710–2719
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127
Vogl SE, Schoenfeld DA, Kaplan BH, Lerner HJ, Engstrom PF, Horton J (1985) A randomized prospective comparison of methotrexate with a combination of methotrexate, bleomycin, and cisplatin in head and neck cancer. Cancer 56:432–442
Vokes EE, Weichselbaum RR, Lippman SM, Hong WK (1993) Head and neck cancer. N Engl J Med 328:184–194
Wanebo HJ, Chougule P, Ready N, Koness RJ, Akerley W, McRae R, Nigri P, Leone L, Webber B, Safran H (1999) Preoperative paclitaxel, carboplatin, and radiation therapy in advanced head and neck cancer (stage III and IV). Semin Radiat Oncol 9:77–84
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93:2325–2327
Williams SD, Velez-Garcia E, Essessee I, Ratkin G, Birch R, Einhorn LH (1986) Chemotherapy for head and neck cancer. Comparison of cisplatin + vinblastine + bleomycin versus methotrexate. Cancer 57:18–23
Zenda S, Onozawa Y, Boku N, Iida Y, Ebihara M, Onitsuka T (2007) Single-agent docetaxel in patients with platinum-refractory metastatic or recurrent squamous cell carcinoma of the head and neck (SCCHN). Jpn J Clin Oncol 37:477–481
Acknowledgments
The Paclitaxel Head and Neck Cancer Study Group was comprised of the following institutions: National Cancer Center Hospital East; Aichi Cancer Center; National Kyushu Cancer Center; Keiyukai Sapporo Hospital; Yokohama City University Hospital; Kobe University Hospital; National Hospital Organization, Tokyo Medical Center; Shizuoka Cancer Center; Kagoshima University Hospital; Yokohama City University Medical Center; Kanagawa Cancer Center; Kanazawa University Hospital; Jichi Medical University Hospital; Hokkaido University Hospital; Osaka City University Hospital; Tokai University Hospital; Kyoto Prefectural University Hospital; Kurume University Hospital; and National Shikoku Cancer Center, Japan. The authors would like to thank all the patients who participated in the clinical trial and all members of the Study Group. This study was also supported by a grant from Bristol-Myers K.K.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tahara, M., Minami, H., Hasegawa, Y. et al. Weekly paclitaxel in patients with recurrent or metastatic head and neck cancer. Cancer Chemother Pharmacol 68, 769–776 (2011). https://doi.org/10.1007/s00280-010-1550-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1550-3