Abstract
Purpose
Although selenium compounds possess chemotherapeutic features by inducing apoptosis in cancer cells with trivial side effects on normal cells, the mechanisms underlying its anti-cancer activity are insufficiently understood at the present. In this study, we investigated the effects of rapamycin on apoptosis induced by seleno-L-methionine (SeMet) or selenite in A549 cells.
Methods
The effects of Se compounds, SeMet and selenite, on cell proliferation, apoptosis and its signaling pathway were investigated in established human adenocarcinoma cell line (A549). Cancer cells were treated with each Se during different periods. Cell apoptosis and signaling molecules were analyzed by flow cytometry (TUNEL method) or immunoblotting, respectively.
Results
SeMet induces reactive oxygen species generation associated with the induction of apoptosis, because pretreatment of cells with N-acetyl-L-cysteine completely blocked SeMet-induced apoptosis. We also found that rapamycin completely suppressed the apoptosis of cells treated by SeMet, but not selenite. SeMet-induced apoptosis is significantly downregulated in combination with PI3 K family inhibitors (LY294002, wortmannin, PI-103, and 3-methyladenine). In addition, ROS generation was included in downstream signaling events associated with the phosphorylation of mTOR, because pretreatment of cells with rapamycin inhibited ROS generation.
Conclusion
These results suggest that SeMet-induced apoptosis is affected by the Akt/mTOR/ROS pathway in A549 cells. Akt serves an anti-survival function in the system of SeMet-treated lung cancer cells, but autophagic signaling remained unsolved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant tumors are a leading cause of death in many countries despite advances in early detection and treatment, and, until now, the chemotherapeutic agents have had insufficient efficacy against tumor cells. Dietary selenium (Se) compounds are promising anti-cancer agents that have shown strong activity against a wide variety of cancer types in vitro and in vivo [1]. Among their compounds, seleno-L-methionine (SeMet) is a naturally occurring amino acid that can provide Se to multiple cellular pools and has potent growth inhibitory and apoptotic activities against multiple human tumor cell lines, including breast, colon, liver, lung, prostate, skin and lymphoid cells [2]. SeMet has also been suggested to have anti-cancer/chemopreventive properties in human clinical studies [3]; however, acute or chronic exposure to high concentrations of SeMet has caused toxicity [4]. Disruption of the PI3 K/Akt pathway by Se has been reported in a variety of cancer cells. For example, PI3 K activity was inhibited by Se-methylselenocysteine (MSC), followed by dephosphorylation of Akt [5]. Methylseleninic acid (MSA) modestly attenuated Akt phosphorylation in LNCaP prostate cancer cells. In contrast, selenite treatment increased the phosphorylation of Akt and p53, but selenite-induced apoptosis was not influenced by chemical inhibitors of either kinase [6]. A combination of selenite and genistein had synergistic effects on apoptosis, through the inhibition of Akt phosphorylation by genistein [7]. These findings support the differential involvement of these protein kinase pathways in regulating apoptosis induction by different forms of Se. The targets and underlying mechanism of anti-cancer action by Se are, therefore, largely unknown.
In general, the PI3 K/Akt/mammalian target of rapamycin (mTOR) pathways promote normal cell growth and proliferation, and their constitutive activation has been implicated in many human cancers [8]. Akt mediates survival and anti-apoptotic signaling, which is activated indirectly by PI3 K. Anti-cancer agents induced caspase-mediated apoptosis associated with the decreased PI3 K/Akt/mTOR signaling axis in cancer cells [6]. Despite its ability to inhibit apoptosis, Akt could not protect against reactive oxygen species (ROS)-mediated cell death but rather sensitized cells to this cell death [9]. The mTOR signaling pathway has critical functions in protein synthesis, controlling cell size and cell cycle progression in response to extracellular and intracellular stimuli. The highly conserved serine/threonine kinase mTOR was found to exist in the form of two distinct protein complexes, mTORC1 and mTORC2, with both fulfilling different molecular functions [10]. Rapamycin and its analogs, such as CCI-779, RAD001 and AP23573, are molecular targeting agents that specifically inhibit mTOR. Rapamycin is of significant interest as a potential anti-cancer drug because many cancers, including lymphoma, pancreatic, colon, prostate, and breast cancers, demonstrate increased mTOR signaling [11]. The inhibition of mTOR decreases the phosphorylation and activation of S6 kinase and 4EBP1, resulting in the inhibition of the translation of critical mRNAs involved in cell cycle progression and, ultimately, cell cycle arrest in the early G1 phase. In addition, rapamycin is the best characterized drug that enhances autophagy [12], which has a synergistic cytotoxic effect with other chemotherapeutic agents in several cancer cell types [13]. Although rapamycin has shown clinical efficacy in a subset of cancers, this mode of drug action does not fully exploit the anti-tumor potential of mTOR targeting in cancer.
ROS play very critical roles in the determination of cell fate by eliciting a wide variety of cellular responses, such as proliferation, differentiation and apoptosis. Although low levels of ROS regulate cellular signal transduction and play important roles in normal cell proliferation, high levels of ROS lead to apoptosis [14]. Extensive studies have indicated that ROS are involved in apoptosis caused by anti-cancer drugs [15]. Several groups have suggested that Se could induce apoptosis in transformed cells through a redox pathway, and ROS was a critical mediator of Se-induced apoptosis [16]. A number of pro-apoptotic and anti-apoptotic proteins are present in the mitochondrial membrane. Oxidative damage to mitochondria is a critical event in apoptosis. The release of cytochrome c from injured mitochondria was shown to activate caspase-3. In addition, evidence has suggested that ROS act as second messengers that are required for downstream signaling effects. ROS generation has been shown to be involved in the Akt signaling pathway [17]. The majority of research on Akt has focused on its role in cell growth promotion, and little is known about its function in cell apoptosis.
We were interested in determining how rapamycin would influence cancer cell death by SeMet, as mTOR has been implicated in both cancer cell death and survival. During the course of these studies, we found that Akt sensitizes A549 cells to ROS-mediated apoptosis. Thus, Akt can be exploited in cancer therapy to selectively kill cancer cells. The results obtained here suggest that the Akt/mTOR axis acts as a self-destructive mechanism in SeMet-treated cancer cells, and that Akt/mTOR inhibitors are cancer cells resistant to SeMet by blocking the apoptosis pathway.
Materials and methods
Reagents
Seleno-L-methionine (SeMet) and sodium selenite were purchased from Sigma (St. Louis, MO, USA). Phosphoinositide 3-kinase (PI3 K) family inhibitors, LY294002 and wortmannin, and free radical scavenger N-acetyl-L-cysteine (NAC) were purchased from Calbiochem (La Jolla, CA, USA). mTOR inhibitor rapamycin was obtained from Wako (Osaka, Japan). Autophagy inhibitor 3-methyladenine (3-MA; Calbiochem) was used. The dual PI3 K/mTOR inhibitor PI-103 was purchased from Calbiochem and dissolved in dimethyl sulfoxide. All other chemicals used in this study are commercially available.
Cell lines and cell culture
A549 cells (wild-type p53, human non-small-cell lung adenocarcinoma) were obtained from the Cell Resource Center for Biomedical Research (Institute of Development, Aging and Cancer, Tohoku University, Japan). Cells were cultured in RPMI 1640 medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). Cells were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
Cell viability assays
Cell viability was evaluated by the Trypan blue exclusion assay, and the cytotoxic effects of each drug were determined using an MTT (WST-8) colorimetric assay kit (Dojindo, Kumamoto, Japan), as described previously [2].
TUNEL assay
Apoptotic cells were assayed by the TUNEL method using the Mebstain apoptosis kit direct (MBL, Nagoya, Japan) for flow cytometric analysis (FACSCalibur; Becton–Dickinson, San Jose, CA, USA). All experiments were conducted in triplicate.
Western blotting
Whole proteins were analyzed by Western blotting as described previously [2]. The following antibodies were used: anti-phospho-mTOR and anti-LC3B (Cell Signaling, Danvers, MA, USA); anti-p62 (MBL); and anti-β-actin (BioVision, Mountain View, CA, USA). HRP-conjugated secondary antibodies, sheep anti-mouse IgG (GE Healthcare, Piscataway, NJ, USA) and goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were also used.
ROS measurement
Intracellular ROS production was measured using 5-(6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCF-DA) as a fluorescent probe (Invitrogen, Carlsbad, CA, USA). Cells were cultured in 6-well plates at 1 × 106 cells/well. Cells were treated with the indicated agents and incubated for 48 h. After incubation, cells were exposed to dyes (2.5 μM) for 30 min at 37°C, harvested, and then analyzed using FACSCalibur. The mean fluorescence intensity (MFI) was used for an index of the ROS level.
Measurement of Akt phosphorylation
Akt protein activation by phosphorylation was assayed with an ELISA (Active Motif, Carlsbad, CA, USA) specific to p-Ser473 Akt and total Akt in HSC-3 and A549 cells, as described previously [18]. Results are expressed as the absorbance at 450 nm.
Statistical analysis
Data are given as the means ± SE. When required, multiple comparisons were made by Scheffe’s test. P values less than 0.05 were regarded as significant.
Results
Inhibition of SeMet-induced apoptosis by rapamycin
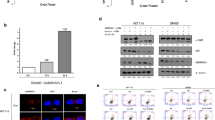
To reevaluate previous results showing the induction of apoptosis by Se compounds [2], we first examined whether SeMet and selenite could induce apoptosis in lung cancer cell line A549. After cells were treated with SeMet (50 μM) or selenite (5 μM) for various lengths of time, the apoptosis rate was evaluated by the TUNEL method. As shown in Fig. 1a, the apoptosis rate of SeMet alone reached 66.5 ± 2.4% after 5 days of incubation, and when cells were treated for 4 days with selenite, the apoptosis rate increased to 39.9 ± 5.4% (Fig. 1b). Our previous results showed that treatment of A549 cells with SeMet activated caspase-3, caspase-8, and caspase-9 in a p53-dependent manner [2].
Inhibition of SeMet-induced apoptosis by rapamycin. A549 cells were treated with a SeMet (50 μM) and b sodium selenite (5 μM) in the presence of rapamycin (10 nM) for 5 or 4 days, followed by the TUNEL assay. Cells pretreated for 1 h with 3-MA (1 mM), LY294002 (LY, 30 μM), wortmannin (Wort, 60 μM), or PI-103 (30 μM), were treated with c SeMet or d selenite without washing, and then subjected to the TUNEL assay. Fluorescence-positive cells, including the apoptotic subpopulation, were quantified. Results are expressed as the means ± SE of triplicate experiments. *P < 0.05 versus SeMet or selenite alone
We next investigated the signaling pathways of PI3 K/Akt/mTOR required for apoptosis induction, using a pharmacological inhibitor of mTOR (rapamycin), a downstream target of Akt. Surprisingly, SeMet-induced apoptosis was markedly inhibited in rapamycin pretreatment (Fig. 1a), whereas no changes were found in apoptosis by selenite/rapamycin (Fig. 1b). PI3 K inhibitor LY294002 was able to significantly inhibit apoptosis by SeMet. Both wortmannin and PI-103 are more effective than LY294002 in blocking apoptosis by SeMet (Fig. 1c). Pretreatment with 3-MA modestly decreased the percentage of apoptotic cells. When cells were treated with selenite, apoptosis was significantly decreased in the presence of LY294002 or PI-103 (Fig. 1d). It is noteworthy that PI3 K family inhibitors were not cytotoxic to the cells at the concentrations used, in the absence of Se compounds. Taken together, these results suggest that SeMet showed different properties from selenite in response to rapamycin.
Phosphorylation of Akt and mTOR by SeMet
The mTOR signaling protein is one of the downstream targets of PI3 K/Akt. To examine the effect of SeMet on Akt or mTOR activation, A549 cells were treated with SeMet for 12 h. Levels of phospho-Akt and phospho-mTOR were examined by ELISA or Western blot using phospho-specific antibodies, respectively. Levels of phospho-Akt were increased after SeMet treatment, whereas constitutive Akt activity of cells was markedly reduced in the presence of LY294002 (Fig. 2a). In addition, SeMet treatment for 48 h clearly increased mTOR activity. This was evidenced by the appearance of phospho-mTOR. In contrast, we observed no signal of phospho-mTOR in selenite-treated cells, whereas the signal of phospho-mTOR was detected in untreated cells (Fig. 2b).
Akt and mTOR phosphorylation by SeMet. a A549 cells were treated with SeMet (100 μM) or LY294002 (30 μM) for 12 h, and fixed. Phospho-Akt and total-Akt were assayed in triplicate, and reactions were measured at OD450 nm. Results are the means ± SE of OD450 values from triplicate experiments. b Cells were treated with SeMet or sodium selenite for 48 h. Total cell lysates were prepared for phospho-mTOR expression, and equal amounts of lysates were loaded for Western blotting. β-Actin was used as a loading control
Involvement of ROS in Se-induced apoptosis
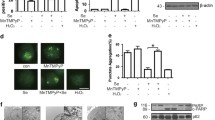
We investigated whether Se compounds could increase the ROS level in A549 cells. Cells were examined for evidence of oxidative stress using a peroxide-sensitive DCF fluorescence assay. ROS accumulation was observed at 48 h of treatment with SeMet or selenite in A549 cells, compared to the control group (Fig. 3). The ROS scavenger NAC at 3 mM abrogated DCF accumulation induced by SeMet or selenite (Fig. 3). In addition, we investigated whether the generation of intracellular ROS is part of the mechanism by which SeMet induces apoptosis in A549 cells. Pretreatment of cells with NAC completely blocked SeMet-induced apoptosis in cells, as well as selenite-induced apoptosis (Fig. 4a, b). Taken together, these results demonstrate that the generation of ROS by SeMet treatment enhances the progress of apoptosis.
SeMet-induced ROS generation. A549 cells were treated with SeMet (50 μM) or sodium selenite (2.5 μM) for 48 h with or without NAC (3 mM). The level of ROS in cells was detected by CM-H2DCF-DA-dependent measurements. The mean fluorescence intensity (MFI) was used for an index of ROS levels. *P < 0.05 versus SeMet or selenite alone
Involvement of ROS in Se-induced apoptosis. A549 cells were treated with a SeMet (5 days) or b sodium selenite (4 days) in the presence of NAC, and then subjected to the TUNEL assay. Fluorescence-positive cells, including the apoptotic subpopulation, were quantified. Results are expressed as the means ± SE of triplicate experiments. c Cells were treated with SeMet for 48 h in the presence of rapamycin. The levels of ROS in cells were detected by CM-H2DCF-DA-dependent measurements. *P < 0.05 versus SeMet or selenite alone
Regulation of intracellular levels of ROS by rapamycin
There are likely multiple proteins and pathways that regulate ROS generation; of special interest is the PI3 K/Akt/mTOR pathway. To evaluate the potential role of the mTOR pathway in the regulation of ROS levels, we used rapamycin to treat A549 cells. Treatment with rapamycin markedly reduced SeMet-induced generation of ROS (Fig. 4c). These results indicated that activation of the PI3 K/Akt/mTOR pathway is required for optimal induction of ROS.
Conversion efficiency of LC3B-I to LC3B-II by SeMet
In order to examine the contribution of autophagy to SeMet-induced apoptosis in A549 cells, autophagy was monitored by measuring the conversion of the cytoplasmic form of LC3B-I to the autophagosomal membrane-bound form of LC3B-II. LC3B-II can be used to estimate the abundance of autophagosomes before they are destroyed through fusion with lysosomes [19]. Lysates of cells treated with SeMet (100 μM) were subjected to Western blot analysis, and processing of LC3B-I to LC3B-II was analyzed. In A549 cells, the level of LC3B-II was slightly increased by incubation with SeMet (48 h) or selenite (24 h), whereas clear bands corresponding to LC3B-II were found in control cells (Fig. 5).
Discussion
Our earlier work showed that different classes of Se compounds induce apoptosis with differential mechanisms in human carcinoma cell lines [2]. In the present study, we have demonstrated that SeMet exposure induced apoptosis through the Akt/mTOR signal transducing pathway, indicated by evidence that mTOR or PI3 K inhibitors completely blocked SeMet-induced apoptosis. In addition, rapamycin significantly reduced ROS generation. Thus, we can conclude that SeMet-induced apoptosis proceeds in a PI3 K/ROS-dependent manner.
Previous study showed that free radicals could cause extensive chemical modifications and alterations in DNA and proteins, including modified bases and sugars, and even strand breaks [20]. The generation of ROS in response to Se compounds has been implicated in the triggering of apoptosis. During selenite-induced apoptosis, cysteine residues in Bax can chemically react with ROS, leading to a change in its conformation and its subsequent translocation to mitochondria [21]. Selenocystine triggers DNA damage that mediates the apoptotic pathway in selected cancer cells [22]. The present study demonstrates that SeMet exposure caused ROS production and cell apoptosis. Using CM-H2DCF-DA susceptible to oxidation by H2O2, we found that both SeMet and selenite mainly induced H2O2 generation, indicating that H2O2 is likely the main ROS involved in SeMet-induced apoptosis. Moreover, pretreatment of A549 cells with the free radical scavenger NAC, which preferentially quenched the hydroperoxide type of ROS, reduced SeMet-induced ROS generation, and apoptosis was completely blocked by NAC (Fig. 4a). Pretreatment with NAC resulted in near complete inhibition of selenite-induced DCF fluorescence (Fig. 4b). Even though the exact mechanism of ROS generated by SeMet is unknown, several sources of ROS generation could exist in cells, including the mitochondrial electron transfer system.
ROS induced by anti-cancer agents may act in an antagonistic manner to anti-apoptotic molecules included in the Akt/mTOR signaling pathway in cancer cells. Of special interest for SeMet-induced apoptosis is the requirement for an active PI3 K/mTOR pathway in the induction of elevated levels of intracellular ROS. Downstream signaling events associated with the phosphorylation of mTOR include ROS generation, because pretreatment of cells with rapamycin inhibited ROS generation (Fig. 4c). A recent report demonstrated that ROS generation in transformed cells required PI3 K/Akt/mTOR activation [23]. The exact role of PTEN in our system, a tumor suppressor and a target of mutations in solid tumors, in the direct regulation of ROS and PI3 K in A549 cells still needs to be determined. Akt kinases play critical roles in regulating growth, proliferation, survival, metabolism, and other cellular activities. In contrast to its well-established survival-promoting role, we found here that Akt also plays a pro-apoptotic role in SeMet-induced apoptosis. In agreement with our study, it is indicated that Akt is not just a single function kinase, and under certain conditions, activation of Akt may be beneficial to cell death. It was shown that Akt activation increases oxidative stress, which in turn further increases Akt phosphorylation and renders cells susceptible to ROS-triggered cell death [9]. In addition, anti-cancer drugs, such as methotrexate, docetaxel, and doxorubicin, can also activate the Akt/Cdk2 pathway to promote, rather than suppress, cell death [24]. In the case of the death receptor pathway, activation of Akt by Fas ligand stimulation leads to apoptosis in epidermal C141 cells [25]. A recent report suggested strongly that the transient activation of Akt supports cell survival, whereas its sustained activation can lead to cellular oxidative stress, eventually resulting in apoptosis through increased Foxo3a expression [26]. Taken together, these data provide novel insights into the molecular consequences of uncontrolled Akt/mTOR activation.
In this study, SeMet increased the phosphorylation level of mTOR at 48 h of incubation in A549 cells, although selenite exposure decreased phospho-mTOR compared to the control (Fig. 2b). Activation of mTOR signaling pathways by SeMet was found to enhance the ability to initiate the apoptosis machinery (Fig. 1a). In our studies, we demonstrated that SeMet treatment caused a transient increase in phosphorylation with consequent activation of Akt, whereas the amount of phospho-Akt was decreased in selenite-treated cells [2]. Recently, it was suggested that apoptosis was effectively induced through autophagy modulation in cancer cells. Sodium selenite increases NB4 cell apoptosis by autophagy inhibition through PI3 K/Akt, and the inhibition of autophagy contributes to the upregulation of apoptosis [27]. MSA is a substrate for reduction to methylselenol by thioredoxin reductase. When thioredoxin reductase levels were reduced using siRNA, there was a clear increase in LC3B-II in cells treated with MSA compared to treatments with MSA or the siRNA alone [28]. The detection of processed LC3B-II by Western blotting has been the mainstay of autophagy detection, although LC3B-II expression levels can vary markedly between different cell types and in response to different stresses. In this study, no evidence of autophagy was confirmed because a higher conversion rate of LC3B-I to LC3B-II could be not observed in response to SeMet and selenite (Fig. 5).
Clinical trials are ongoing with rapamycin and its analogs in various tumor types. In addition to direct anti-tumor effects, rapamycin specifically inhibits mTORC1, but not mTORC2, by disrupting the interaction between raptor and mTOR. The mTORC2 complex is responsible for the phosphorylation of Akt. It was reported that prolonged treatment with rapamycin resulted in the inhibition of mTOR and increased Akt phosphorylation at Ser473, suggesting that secondary activation of Akt may occur as a consequence of mTORC2 activation [29]. In addition, a recent report suggested that mTOR inhibition by rapamycin might be related to the feedback activation of Akt and that this can cause hyper-activation of Akt and resistance to apoptosis [30]. Although the efficacy of rapamycin as a single agent is limited in most tumor types, anti-cancer effects of conventional chemotherapeutic agents are enhanced in combination with rapamycin. For example, rapamycin interacts with 5-fluorouracil in a synergistic manner in scirrhous gastric cancer cells by activation of the apoptosis signal [31]; however, an antagonistic effect was found for the combination of mTOR inhibitors with anti-cancer drugs (paclitaxel, gemcitabine, irinotecan, and oxaliplatin). We also found that low doses (10 nM) of rapamycin completely protected against SeMet-induced apoptosis (Fig. 1a). In this regard, rapamycin may act as one of the anti-apoptotic components of the Akt/mTOR signaling pathway. Conversely, the Akt/mTOR pathway may represent a common pro-apoptotic mechanism utilized by cancer cells.
Wortmannin acts as a potent inhibitor of PI3 K; however, it is nonspecific as it also elicits a strong inhibitory effect on MAPK. LY294002 is a commonly used pharmacologic inhibitor of PI3 K, where it acts on the ATP binding site of the PI3 K enzyme, thus selectively inhibiting the PI3 K/Akt nexus. PI-103, the first synthetic multi-targeted compound, simultaneously inhibits PI3 Kα and mTOR. The early and still frequently employed inhibitors, wortmannin and LY294002, have significant limitations as chemical tools, although they were valuable in the past. PI-103 exhibited advantages over wortmannin and LY294002, with excellent potency and selectivity [32]. In this study, PI-103 was more effective than LY294002 and wortmannin in blocking apoptosis by SeMet (Fig. 1c). Although 3-MA is widely used as an inhibitor of autophagy, 3-MA suppresses the invasion of HT1080 cells, independently of autophagy inhibition, through the inhibition of type I and II PI3 Ks and possibly other molecules [33].
In summary, a host of dietary factors can influence various cellular processes and thereby potentially influence the overall cancer risk and tumor behavior. In many cases, these factors suppress cancer by stimulating programmed cell death. The present study provides insight into the role of ROS, Akt, and mTOR signaling in apoptotic death by SeMet in A549 lung adenocarcinoma cells. Better understanding of the mechanism of action of SeMet could potentially facilitate the clinical development of SeMet for malignant tumors.
References
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Suzuki M, Endo M, Shinohara F, Echigo S, Rikiishi H (2010) Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother Pharmacol 66:475–484
Fakih MG, Pendyala L, Brady W, Smith PF, Ross ME, Creaven PJ, Badmaev V, Prey JD, Rustum YM (2008) A Phase I and pharmacokinetic study of selenomethionine in combination with a fixed dose of irinotecan in solid tumors. Cancer Chemother Pharmacol 62:499–508
Schrauzer GN (2000) Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr 130:1653–1656
Unni E, Koul D, Yung WK, Sinha R (2005) Se-methylselenocysteine inhibits phosphatidylinositol 3-kinase activity of mouse mammary epithelial tumor cells in Vitro. Breast Cancer Res 7:699–707
Hu H, Jiang C, Li G, Lu J (2005) PKB/AKT and ERK regulation of caspase-mediated apoptosis by methylseleninic acid in LNCaP prostate cancer cells. Carcinogenesis 26:1374–1381
Zhao R, Xiang N, Domann FE, Zhong W (2009) Effects of selenite and genistein on G2/M cell cycle arrest and apoptosis in human prostate cancer cells. Nutr Cancer 61:397–407
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657
Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N (2008) Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 14:458–470
Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124:471–484
Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N (2006) mTOR signaling: Implications for cancer and anticancer therapy. Br J Cancer 94:195–199
Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N, Yahalom J (2005) Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res 65:11061–11070
Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, Meric-Bernstam F (2004) Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res 10:7031–7042
Hussain SP, Hofseth LJ, Harris CC (2003) Radical causes of cancer. Nat Rev Cancer 3:276–285
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W (2000) Superoxide dismutase as a target for the selective killing of cancer cells. Nature 407:390–395
Hu D, Liu Q, Cui H, Wang H, Han D, Xu H (2005) Effects of amino acids from selenium-rich silkworm pupas on human hepatoma cells. Life Sci 77:2098–2110
Koshikawa N, Hayashi J, Nakagawara A, Takenaga K (2009) Reactive oxygen species-generating mitochondrial DNA mutation up-regulates hypoxia-inducible factor-1α gene transcription via phosphatidylinositol 3-kinase-Akt/protein kinase C/histone deacetylase pathway. J Biol Chem 284:33185–33194
Suzuki M, Shinohara F, Rikiishi H (2008) Zebularine-induced reduction in VEGF secretion by HIF-1α degradation in oral squamous cell carcinoma. Mol Med Rep 1:465–471
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 21:5720–5728
Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA (2009) Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis 30:2–10
Huang F, Nie C, Yang Y, Yue W, Ren Y, Shang Y, Wang X, Jin H, Xu C, Chen Q (2009) Selenite induces redox-dependent Bax activation and apoptosis in colorectal cancer cells. Free Radic Biol Med 46:1186–1196
Chen T, Wong YS (2009) Selenocystine induces reactive oxygen species-mediated apoptosis in human cancer cells. Biomed Pharmacother 63:105–113
Kim JH, Chu SC, Gramlich JL, Pride YB, Babendreier E, Chauhan D, Salgia R, Podar K, Griffin JD, Sattler M (2005) Activation of the PI3 K/mTOR pathway by BCR-ABL contributes to increased production of reactive oxygen species. Blood 105:1717–1723
Maddika S, Ande SR, Wiechec E, Hansen LL, Wesselborg S, Los M (2008) Akt-mediated phosphorylation of CDK2 regulates its dual role in cell cycle progression and apoptosis. J Cell Sci 121:979–988
Lu B, Wang L, Stehlik C, Medan D, Huang C, Hu S, Chen F, Shi X, Rojanasakul Y (2006) Phosphatidylinositol 3-kinase/Akt positively regulates Fas (CD95)-mediated apoptosis in epidermal Cl41 cells. J Immunol 176:6785–6793
Van Gorp AG, Pomeranz KM, Birkenkamp KU, Hui RC, Lam EW, Coffer PJ (2006) Chronic protein kinase B (PKB/c-akt) activation leads to apoptosis induced by oxidative stress-mediated Foxo3a transcriptional up-regulation. Cancer Res 66:10760–10769
Ren Y, Huang F, Liu Y, Yang Y, Jiang Q, Xu C (2009) Autophagy inhibition through PI3 K/Akt increases apoptosis by sodium selenite in NB4 cells. BMB Rep 42:599–604
Honeggar M, Beck R, Moos PJ (2009) Thioredoxin reductase 1 ablation sensitizes colon cancer cells to methylseleninate-mediated cytotoxicity. Toxicol Appl Pharmacol 241:348–355
Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W (2010) Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 6:366–377
Shi Y, Yan H, Frost P, Gera J, Lichtenstein A (2005) Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 4:1533–1540
Matsuzaki T, Yashiro M, Kaizaki R, Yasuda K, Doi Y, Sawada T, Ohira M, Hirakawa K (2009) Synergistic antiproliferative effect of mTOR inhibitors in combination with 5-fluorouracil in scirrhous gastric cancer. Cancer Sci 100:2402–2410
Workman P, Clarke PA, Raynaud FI, van Montfort RL (2010) Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res 70:2146–2157
Ito S, Koshikawa N, Mochizuki S, Takenaga K (2007) 3-Methyladenine suppresses cell migration and invasion of HT1080 fibrosarcoma cells through inhibiting phosphoinositide 3-kinases independently of autophagy inhibition. Int J Oncol 31:261–268
Acknowledgments
We thank Mr. D. Mrozek for editing the manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research (20659309) from the Japan Society for the Promotion of Science and (22791948 and 21791967) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, M., Endo, M., Shinohara, F. et al. Rapamycin suppresses ROS-dependent apoptosis caused by selenomethionine in A549 lung carcinoma cells. Cancer Chemother Pharmacol 67, 1129–1136 (2011). https://doi.org/10.1007/s00280-010-1417-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1417-7