Abstract
Purpose
H1 is a novel derivative of tetrandrine (Tet). Here we investigate the ability of H1 to reverse P-glycoprotein (Pgp)-mediated multidrug resistance (MDR) and its mechanisms.
Methods
KBv200, MCF-7/adr and their parental sensitive cell lines KB, MCF-7 were used for reversal study. The intracellular accumulation and efflux studies with Pgp substrates of doxorubicin and rhodamine 123 were determined by flow cytometry. The expression of Pgp was investigated by Western blot and RT-PCR analysis. ATPase activity of Pgp was performed by Pgp-Glo™ assay systems. The ubiquitination level of Pgp was determined by immunoprecipitation analysis. The effect of ERK1/2 on Pgp expression in KBv200 cells were investigated by RNA interference.
Results
H1 significantly potentiated the sensitivity of Pgp substrates in KBv200 and MCF-7/adr cells, but not in parental cells KB and MCF-7. H1 inhibited Pgp expression in KBv200 cells in a dose-dependent manner, but had no effect on MDR1 expression. Further studies showed that H1 prompted the degradation of Pgp and decreased Pgp protein half-life by enhancing the ubiquitination of Pgp, which may be related to downregulated MEK-ERK signal pathway. We also found H1 inhibited ATPase activity of Pgp in a dose-dependent manner.
Conclusions
H1 is an effectively and potential agent in reversing Pgp-mediated MDR by inhibiting the transport function and expression of Pgp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, multidrug resistance (MDR) to anti-cancer drugs is a major obstacle to the effective treatment of cancer. One of the underlying mechanisms of MDR is cellular overexpression of P-glycoprotein (Pgp) which encoded by the MDR1 gene. Pgp is a transmembrane protein and functions as an ATP-dependent drug transporter that transports the intracellular drugs out of the cells [1]. As such, the intracellular drugs in the MDR cancer cells could be kept at sub-lethal level, by which cancer cells circumvent the effective attack by the anti-cancer drugs [1, 2]. Inhibition of the transport function and expression of Pgp is one of the effective ways to reverse cancer drug resistance.
Tetrandrine (Tet) is a bisbenzylisoquinoline, which is the main active component in the root Stephania tetrandra S. Moore [3]. The reverse activity of Tet was associated with an increase of the intracellular drug accumulation induced by direct binding to Pgp. Tet had potent reversal effect on Pgp-mediated MDR in vitro and in vivo [4, 5]. Our previous studies reported that 5-Bromotetrandrine (BrTet), a bromized derivative of Tet, exhibited more potent reversal effect than Tet in vitro. It sensitized several MDR cells to various anti-cancer drugs in Pgp-mediated MDR cells and significantly enhanced the anti-cancer activity of Dox in nude mice bearing resistant KBv200 xenografts [6, 7]. Phase I clinical trial of BrTet has been completed, and now phase II clinical trail is under progress. H1 was a novel derivative of tetrandrine, which was synthesized by Prof Wang. Our preliminary screening results showed that the reversal activity of H1 was more potent than Tet and BrTet. It significantly potentiated the cytotoxicity of anti-cancer drugs in the Pgp-overexpressing cells, but not in the Pgp-negative parental cells. The present study is undertaken to further evaluate the reversal effect of H1 on Pgp-mediated MDR along with its ability to inhibit the transport function and expression of Pgp.
Materials and methods
Chemicals and reagents
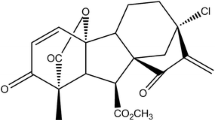
H1 was synthesized by Prof Wang. The powder of H1 with a purity of >99% was obtained by chromatography, and freshly solved in dimethyl sulfoxide (DMSO) before use. The chemical structure showed in (Fig. 1b), molecular formula: C27H40N2O6Br, molecular weight: 690. PD98059, U0126 was purchased from Cell Signaling technology, Inc. Paclitaxel (Tax), doxorubicin (Dox), vincrinstine (Vcr), verapamil (Vrp), 1-(4, 5-dimethylthiazol-2-yl)-3, 5-diphenyformazan (MTT) and other chemicals were purchased from sigma chemical Co. (St. Louis, MO). MDR1/Pgp antibody was a product of Abcam Biotechnology, Inc. β-actin antibody was purchased from Santa Cruz Biotechnology (USA). MEK1/2, p-MEK1/2, p-ERK1/2, ERK1/2, Ubiquitin antibodies were obtained from Cell Signaling technology, Inc., RT-PCR kit and Pgp-Glo™ assay systems were purchased from Promega (Madison, WI, USA).
Cell lines and cell culture
The MDR cell lines KBv200 and MCF-7/adr were generously provided by Professor Chen Xiaoguang (Chinese Academy of Medical Sciences, Beijing). KBv200 and MCF-7/adr cells, two classical multidrug resistant human cell lines with high level of Pgp expression which was the main cause for inducing MDR, were derivate from their parental sensitive KB cells and MCF-7 by stepwise exposure to increasing doses of vincristine (Vcr) or doxorubicin (Dox) [8, 9]. In comparison with KB, MCF-7 cells were approximately 100-fold resistant to Vcr and Dox, respectively. All the cell lines were all grown in RPMI1640 (GibcoBRL) medium supplemented with 10% heat-inactivated new-born calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin. To maintain drug resistance, 200 nM Vcr and 200 nM Dox were added to the culture of KBv200 and MCF-7/adr cells, respectively. All experiments were performed after 7–10 days of incubation in drug-free medium.
MTT assay
MTT assay was used to assess cytotoxicity of H1 [10]. In detail, cells were seeded in 96-well multiplates. After an overnight incubation (37°C with 5% CO2), various concentrations of H1 were added into the wells and incubated for another 72 h. Thereafter, 100 μl of 0.5 μg/ml MTT was added to each well after the withdrawal of the culture medium and this was then incubated for an additional 4 h. The resulting formazan was dissolved in 150 μl DMSO after aspiration of the culture medium. Plates were placed on a plate shaker for 30 min and read immediately at 570 nm using a microplate reader (Bio-Rad Model 450). The IC50 values (the concentration resulting in 50% inhibition of cell growth) were calculated by Graphpad Prism 6.0 software. According to the results of cytotoxicity assay, noncytotoxic concentrations of H1 were selected to determine the reverse MDR effect. Vrp was used as a positive control. MDR reversal effect of H1 is expressed as fold reversal (FR) which calculated by dividing the IC50 for the cells to Vcr, Dox and Tax in the absence of H1 by that obtained in the presence of H1. FR = IC50 anti-cancer drug alone/IC50 anti-cancer drug + modulator [6].
Flow cytometric analysis
The effects of H1 on the cellular accumulations of Dox and rhodamine 123 were measured by flow cytometry. Briefly, 5 × 105 cells were incubated in 6-well plates and allowed to attach overnight. The cells were then treated with H1 of desired concentrations (0.125, 0.25, 0.5 μM) and vehicle at 37°C for 3 h. Then 10 μM Dox and rhodamine 123 were added and further incubated for another 3 or 0.5 h, respectively. Following this incubation, the cells were collected and washed twice with ice-cold PBS buffer. Finally, the cells were resuspended in PBS buffer for flow cytometric analysis (Beckman Coulter, Cytomics FC500, USA). Vrp was used as a positive control [11, 12]. The relative values were identified by dividing the fluorescence intensity of each measurement by that of control cells.
Rhodamine 123 efflux assay was also detected by flow cytometry. The ability of H1 to maintain inhibition of Pgp-mediated efflux after its removal from the incubation medium was assessed by using Pgp substrate rhodamine 123. KBv200 cells were seeded in 6-well plates at a density of 5 × 105 cells and allowed to attach overnight. Vrp and various concentration of H1 added into the cells and incubated for 1 h, then rhodamine 123 (10 μM) was additional added in the cells incubated for another 0.5 h at 37°C in 5% CO2. The cells were collected and washed with fresh medium and resuspended in normal growth medium without H1 or rhodamine 123. Samples were incubated at 37°C, and aliquots removed at 10, 20, 30, 60 and 120 min, respectively. Then the cells were centrifuged and washed in ice-cold PBS. At last, the cells were resuspended in 1 ml PBS and analyzed by flow cytometry. Fluorescence was measured from 104 cells and cell-associated fluorescence as a percentage of T0 was plotted against time [13].
ATPase assay
The changes of ATPase activity were determined by Pgp-Glo™ assay systems (Promega, USA). Sodium orthovanadate (Na3VO4) was used as a Pgp ATPase inhibitor. The inhibitory effects of H1 were determined against a Vrp-stimulated Pgp ATPase activity. Various concentrations of H1 diluted with assay buffer were incubated in 100 μM Vrp, 5 mM MgATP and 25 μg recombinant human Pgp membranes at 37°C for 40 min. Luminescence was initiated by ATP detection buffer. After incubated at room temperature for 20 min to allow luminescent signal to develop, the untreated white opaque 96-well multiplates (corning, USA) was read on luminometer (SpectraMax M5, molecular devices, USA). The changes of relative light units (△RLU) were determined by comparing Na3VO4-treated samples with H1 and Vrp combination-treated groups.
Western blot analysis
Cells were harvested and rinsed twice with PBS and lyzed in denaturing lysis buffer (Applygen Technologies Inc. China) for 30 min on ice, centrifuged 12,000 g for 15 min at 4°C. Protein concentrations were determined by BCA assay. Equal quantities (40 μg of protein) of cell extract were resolved by 10% SDS–PAGE, the resolved protein were electrophoretically transferred to PVDF membrane and blocked with 5% fat-free dry milk in TBST for 1.5 h at room temperature. The membrane was immunoblotted with first antibody in 5% milk TBST, at 4°C overnight. Membranes were washed three times, incubated with secondary antibodies for 2 h at room temperature and washed three times before detection. The membranes were subsequently developed using ECL (FuJiFilm, Japan) reagent (Applygen Technologies Inc. China) and exposed to film according to the manufacturer’s protocol.
Immunoprecipitation analysis
KBv200 cells were harvested and lyzed in NET buffer (Applygen Technologies Inc. China) for 30 min on ice, centrifuged 12,000 g for 30 min at 4°C. Protein concentrations were determined by BCA assay. Identical amounts (2 mg of protein) were immunoprecipitated with 1 μg Pgp antibody by overnight incubations at 4°C after adjusting the volumes to 1.0 ml with cold NET buffer. The immune complexes were precipitated with Protein-A Sepharose CL-4B (GE Healthcare Inc. USA) and washed three times with TNT buffer (Applygen Technologies Inc. China), once with NET buffer and once with PBS. Immunoprecipitated proteins were detected by Western blot assay using anti-ubiquitin polyclonal antibody [14].
RT-PCR analysis
KBv200 cells were seeded in 6-well plates at a density of 5 × 105 cells and allowed to attach overnight, then cultured with or without H1 (0.125, 0.25, 0.5 μM, respectively) for 48 h. Total RNA was extracted by the guanidine isothiocyanate/phenol/chloroform method. The integrity and purity of the RNA were checked by UV Spectrophotometer for OD260 and OD280, then reverse transcripted from mRNA to cDNA using the RT-PCR kit (Promega, WI, USA). The following primers were used for RT-PCR: MDR1 (286 bp) sense 5′- AGA AGG TTC TGG GAA GA TCGC-3′, anti-sense 5′-ATG TCC TTT TCC AGC ACC TC-3′; β-actin (529 bp) sense 5′- TGT TTG AGA CCT TCA ACA CCC-3′, anti-sense 5′-AGC ACT GTG TTG GCG TAC AG-3′. The PCR profile was as follows: 10 min at 95°C, followed by 30 cycles of 30 s at 95°C and 1 min at 60°C. The PCR product was separated by 1% agarose gel electrophoresis, and the gels were stained using ethidium bromide and viewed by UV transillumination.
RNA inference
siRNAs specific for human ERK1 and ERK2 genes, and the nonspecific negative control siRNA with or without FAM labeled were purchased from Invitrogen. KBv200 cells were seeded in 6-well plates to reach 30–50% confluent and transfected with siRNA for ERK1 and ERK2 using Lipofectamine 2000 (Invitrogen) in antibiotics and serum-free media, according to the manufacturer’s instructions. After 48 h for gene knockdown, KBv200 cells were collected for Western blot analysis.
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis of the data was performed using the one-way ANOVA. P < 0.05 was considered statistically significant.
Results
Characterization of Pgp-overexpressing and corresponding parental cell lines
To characterize Pgp-overexpressing and corresponding parental cell lines, we first detected Pgp expression by Western blot in two pairs of cell lines. As shown in Fig. 2a, b, expression of Pgp in KB and MCF-7 was almost undetectable; while Pgp expression in KBv200 and MCF-7/adr cells were significantly higher compared with their corresponding parental cell lines. Then, the multidrug resistant potency of KBv200 and MCF-7/adr cells to Vcr, Dox, Tax was further evaluated by MTT assay. The reversal fold is expressed as ratio of IC50 between Pgp-overexpressing and corresponding parental cell lines. As shown in Tables 1 and 2, KBv200 cells displayed 34.2-, 75.3- and 30.1-fold resistance to Vcr, Dox, Tax, respectively, compared with KB cells. MCF-7/adr cells exhibited 70.5-, 112.9- and 34.8-fold resistance to Vcr, Dox, Tax, respectively, compared with MCF-7 cells.
Characteristics of tumor cell lines used in this study and the cytotoxicity of H1 in these cell lines. Pgp expression level in KB vs. KBv200 cells (a); Pgp expression level in MCF-7 vs. MCF-7/adr cells (b); KB and KBv200 cells (c); MCF-7 and MCF-7/adr cells (d) were exposed to the indicated concentration of H1 for 72 h. Each point represents the mean ± SD of three determinations. Each experiment was performed in three times
Reversal effect of H1 on Pgp-mediated MDR
First, we examined cytotoxicity of H1 alone in different cell lines by using MTT assay. As showed in Fig. 2c, d, the results indicated that H1 at 2–10 μM exerted considerable cytotoxicity. The MDR cell lines, KBv200 and MCF-7/adr showed the same sensitivity to H1 as their parental cells. More than 90% of cells were survival at the concentration of 0.5 μM H1 in all cells. Based on the results of cytotoxicity assay above, we choose H1 of 0.125, 0.25, 0.5 μM to evaluate the reversal activity. As shown in Table 1, H1 significantly decrease the IC50 values for 3 anti-cancer drugs in KBv200 cells in a dose-dependent manner, but not in KB cells. At the concentration of 0.5 μM, H1 completely reversed the resistance of KBv200 cells to Vcr, Dox and Tax. The reversal fold was 54.0, 28.4, 50.1, respectively, and its potency was greater than that of Vrp. In addition, H1 also actively reversed Pgp-mediated resistance in MCF-7/adr. The results in Table 2 showed that H1 exhibited appreciable reversal of Vcr, Dox and Tax resistance in MCF-7/adr cells in a dose-dependent manner. A concentration 0.5 μM of H1 completely reversed MCF-7/adr cells’ resistance to Dox, and partially reversed MCF-7/adr cells’ resistance to Vcr and Tax. The reversal folds were 24.8, 126.8 and 10.1, respectively. To evaluate substrate specificity of transporter, cisplatin that is not a substrate of Pgp was selected as a control. H1 did not significantly alter the IC50 values of cisplatin in MDR and drug-sensitive cells. These results suggest that H1 significantly potentiated the cytotoxicity of anti-cancer drugs in the Pgp-mediated MDR cells.
H1 enhances the accumulation of Dox and Rhodamine 123 in MDR cells overexpressing Pgp
The results above indicated that H1 could reverse Pgp-mediated MDR. In order to ascertain the mechanism of H1 on the function of Pgp, we determined the intracellular levels of Dox and rhodamine 123 were measured in the absence or presence of H1 in both KB and KBv200 cells by flow cytometry. In the absence of H1, accumulation of Dox and rhodamine 123 in KBv200 cells was much lower than in sensitive KB cells. When the cells were preincubated with H1 for 3 h, H1 significantly increased Dox and rhodamine 123 accumulation in KBv200 cells in a dose-dependent manner, but not in sensitive KB cells. Fig. 3a illustrated that H1 enhanced the accumulation of Dox. The intracellular accumulation of Dox was enhanced 2.0-, 2.6- and 3.7-fold in KBv200 cells; 1.0-, 1.1- and 1.1-fold in KB cells in the presence of 0.125, 0.25, or 0.5 μM of H1, respectively. As depicted in Fig. 3b, rhodamine 123 fluorescence values were enhanced 2.0-, 7.8-, 28.7-fold in KBv200 cells; 1.0-, 0.9-, 1.0-fold in KB cells at H1 varying from 0.125 to 0.5 μM. These results demonstrated that H1 was able to interfere with Pgp-mediated transport.
H1 interfered the function of Pgp. Intracellular accumulation of Dox (a) and rhodamine 123 (b) in KB and KBv200 cells The cells were incubated with H1 (0.125, 0.25, 0.5 μM) at 37°C for 3 h, then 10 μM Dox and rhodamine 123 was added for another 3-h incubation. Intracellular fluorescence was analyzed by flow cytometry. Control cells that were not exposed to any H1, and Vrp (10 μM) were used as positive control. Data were shown as mean ± SD of three independent experiments, *P < 0.05; **P < 0.01 versus control group, respectively. c Inhibition of rhodamine 123 efflux from KBv200 cells by H1 and Vrp. After 1 h in the presence of rhodamine 123 (10 μM) and H1 (0.125, 0.25, 0.5 μM) or Vrp (10 μM), the cells were washed and incubated in fresh medium for indicated times. d H1 inhibited Vrp-stimulated ATPase activity of Pgp. Luminescent Pgp ATPase assay was performed according to Pgp-Glo™ Assay System instruction. Each point represents the mean ± SD of three independent experiments
Effect of H1 on Pgp-mediated efflux of Rhodamine 123
The ability of H1 to inhibit Pgp-mediated efflux and its duration of action was investigated using Pgp substrate rhodamine 123 and resistant cell line KBv200. Figure 3c illustrated clearly that H1 inhibited the efflux of rhodamine 123 from the KBv200 cells and the H1 remains effective when it was excluded from the efflux medium. More than 90% of the cellular rhodamine 123 was retained at 30 min when H1 was present at 0.5 μM concentration. In the presence of 0.125, 0.25 and 0.5 μM of H1, 20.4, 29.6 and 38.7% of the cellular rhodamine 123 was retained at 2 h, respectively. In contrast, >90% of the rhodamine 123 was effluxed by 1 h after incubated with Vrp at 10 μM. The result displayed a good concentration-dependent effect on Pgp-mediated efflux.
Effect of H1 on ATPase activity of Pgp
The efflux function of Pgp is coupled to ATP hydrolysis by the ATPase which could be activated in the presence of Pgp substrates or modulators. To assess the effects of H1 on the ATPase activity of Pgp, we measured Pgp-mediated ATP hydrolysis with various concentrations of H1. As shown in Fig. 3d, H1 inhibited Vrp-stimulated ATPase activity of Pgp in a dose-dependent manner.
Effect of H1 on the expression of Pgp and MDR1
Pgp-mediated MDR can be reversed either by inhibiting their transport function or by decreasing protein expression level of Pgp. To study the effect of H1 on Pgp expression, KBv200 cells were treated with H1 (0.125, 0.25, 0.5 μM) for 48 h and the expression level of Pgp was determined by Western blot analysis. As shown in Fig. 4a, Pgp expression decreased markedly in a does-dependent manner. The result demonstrated that H1 significantly inhibited Pgp expression.
H1 inhibited the expression of Pgp through enhancing the degradation of Pgp. After treated with desired concentrations of H1 for 48 h, the mRNA and protein expression level of Pgp in KBv200 cells treated with or without H1 was determined by RT-PCR and Western blot analysis, respectively (a, b). KBv200 cells were exposed to 100 μg/ml cycloheximide (CHX, known as protein synthesis inhibitor) with or without H1 (0.5 μM) for 12, 18, 24 h to block protein synthesis. The cells were collected for Western blot analysis (c). Effect of H1 on the ubiquitination of Pgp. KBv200 cells were treated for 48 h with or without H1 (0.5 μM). Cell lysates were immunoprecipitated with 1 μg of anti-Pgp polyclonal antibody, followed by Western blotting with a monoclonal anti-ubiquitin antibody (d). A representative result is shown from at least three independent experiments. *P < 0.05; **P < 0.01 versus control group, respectively
The MDR1 gene encodes the drug efflux pump Pgp, whose overexpression is associated with the development of MDR, and the overexpression of Pgp is primarily regulated at the transcriptional level. Given that H1 decreased Pgp expression, we next investigated whether H1 would affect the transcription of MDR1 by using semi-quantitative RT-PCR. After treatment of KBv200 cells with H1 (0.125, 0.25, 0.5 μM) for 48 h, the MDR1 expression level did not alter significantly (Fig. 4b).
Effect of H1 on the degradation of Pgp
Above results indicated that H1 significantly inhibited Pgp expression but did not affect the transcriptional level of MDR1. It suggests that H1 might affect the degradation of Pgp. To test this possibility, we determined the half-life of Pgp protein by the introduction of cycloheximide, a known protein synthesis inhibitor. In presence of H1, the Pgp protein had a shorter half-life (13.5 h) than that (18.3 h) of the control (Fig. 4c). By the way, we also determined the ubiquitination level of Pgp in KBv200 cells with or without H1. As shown in (Fig. 4d), treatment with H1 increased the ubiquitination of Pgp. These results demonstrated that H1 could prompt Pgp degradation through enhancing the ubiquitination of Pgp.
Effect of H1 on the blockade of MEK-ERK signal pathway
MEK-ERK signal pathway involves inhibition of the Pgp expression through the ubiquitination of Pgp in MCF-7/adr cells [15]. To determine whether decreased Pgp expression by H1 was related to the change MEK-ERK pathway, after KBv200 cells treated with desired concentration of H1 for 48 h, total and phosphorylated forms of MEK1/2 and ERK1/2 were measured by Western blot analysis. As shown in Fig. 5a, the phosphorylated MEK1/2 and ERK1/2 were significantly decreased in a dose-dependent manner, but the total MEK1/2 and ERK1/2 were not significantly changed. The results showed that H1 significantly down-regulate MEK-ERK pathway. Subsequently, to further understand the role of ERK1/2 on the regulation of Pgp expression. We tested Pgp expression in KBv200 by using RNA interference to knockdown ERK1 and ERK2 genes. The result showed in Fig 5b, nonspecific-negative control siRNA had no effect on protein expression. When ERK1 or ERK2 was interfered, the protein level of Pgp was decreased. By the way, we also detected the effect of PD98059 and U0126 (known as MEK inhibitors) on Pgp expression in KBv200 cells. As shown in Fig 5c, PD98059 and U0126 also inhibited Pgp expression. Based on these findings, it suggested us that H1 inhibited Pgp expression may be involved in blockade of MEK-ERK pathway.
Effect of H1 on MEK-ERK signal pathway. After treated with H1 for 48 h, the protein expression levels of p-MEK1/2, MEK1/2, p-ERK1/2 and ERK1/2 in KBv200 cells was measured by Western bolt analysis (a). Knockdown ERK1 and ERK2 genes by using RNA interference for 48 h, KBv200 cells were collected for Western blot analysis (b). KBv200 cells were exposed of PD98059 (50 μM) and U0126 (10 μM), known as MEK inhibitors, for 48 h. Equal amounts of cells lysates were used for Western blot analysis. A representative result is shown from at least three independent experiments, **P < 0.01 versus control group
Discussion
Pgp-mediated MDR as known classic MDR, which was identified in the 1970s, is a well-characterized experimental phenomenon [16]. The overexpression of Pgp in cancer cells results in the failure of cancer chemotherapy treatment. To circumvent MDR, much effort has focused on finding Pgp inhibitors to effectively reverse Pgp-mediated MDR. H1, a novel derivate of Tet, was synthesized by Prof Wang. Preliminary screening results showed that the reversal activity of H1 was more potent than BrTet (data not shown). In the present study, we found H1 significantly potentiated the cytotoxicity of anti-cancer drugs in the Pgp-mediated MDR cells (Tables 1 and 2). It interfered with the intracellular accumulation and efflux function of Pgp. Moreover, H1 inhibited Vrp-stimulated ATPase activity of Pgp. H1 decreased Pgp expression, but had no effect on MDR1 expression. Further studies showed that H1 promoted the degradation of Pgp and down-regulated the MEK-ERK pathway. Our results thus show H1 is able to effectively reverse Pgp-mediated MDR by inhibiting transport function and expression of Pgp.
Pgp has a basal ATPase activity and can be activated in the presence of its substrates [17]. One of the important roles of Pgp is to pump out anti-cancer drugs at the expense of ATP hydrolysis. Our results showed that H1 significantly increased intracellular accumulation of Dox and rhodamine 123 and inhibited the Pgp-mediated efflux of rhodamine 123 in KBv200 cells with a dose-dependent manner (Fig. 3a, b, c). The transport function of Pgp is coupled to ATP hydrolysis by the ATPase [18]. Further studies found H1 could inhibit Vrp-stimulated ATPase activity of Pgp (Fig. 3d). These data indicated that H1 could reverse Pgp-mediated MDR by interfering Pgp transport function, and so that increase the cellular concentration of anti-cancer drugs. In addition, Pgp-overexpressing MDR cells were not resistant to H1. It suggested that H1 might not be a substrate of Pgp.
Pgp-mediated MDR could be reversed either by inhibiting their transport function or by decreasing protein expression level of Pgp. Subsequently, we found that H1 could significantly down-regulate Pgp expression. The MDR1 gene encodes Pgp, and overexpression of Pgp is primarily regulated at the transcriptional level. RT-PCR results showed that mRNA expression of MDR1 was not affected (Fig. 4a, b). Given these above findings, it suggests that H1 might affect the degradation of Pgp. At steady state, Pgp is located in the plasma membrane and undergoes endocytosis and recycling [19]. What is more, studies showed that proteosome, but not lysosome, involved in the Pgp turnover. It presented that ubiquitination of the Pgp implicated in regulating its turnover and function [15]. On the basis of above findings, we determined the half-life of Pgp protein by cycloheximide. In presence of H1, the Pgp protein had a shorter half-life (13.5 h) than that (18.3 h) of the control (Fig. 4c). By the way, we also determined the ubiquitination level of Pgp in KBv200 cells with or without H1. As shown in Fig. 4d, treatment with H1 increased the ubiquitination of Pgp. These studies indicated that suppressing the expression of Pgp by enhancing its degradation might be another strategy to effective modulation of Pgp-mediated MDR.
Up to now, the regulatory mechanisms underlying the expression of Pgp have not yet been well clarified. Some studies presented that mitogen-activated protein kinases (MAPK) pathway was associated with regulatory of Pgp expression in Pgp-mediated MDR [20, 21]. Adenoviral transduction of JNK has been shown to down-regulate Pgp expression [22]. SB203580, a p38MAPK inhibitor, has been shown to decrease Pgp expression levels in DU145 spheroids and Vcr-resistant murine leukemia L1210/Vcr cells [23]. Recently, some groups observed that activation of the MEK-ERK-RSK pathway enhanced Pgp expression, while knockdown ERK and RSK down-regulated Pgp expression in MDR cells. MEK inhibitor U0126 was found to potently down-regulate Pgp expression. But the transcriptional levels of MDR1 were not affected. Further studies indicated that a blockade of the MEK-ERK pathway suppressed Pgp expression by promoting its degradation [14]. Given that the relationship between MAPK pathway and regulatory of Pgp expression, we determined the expression of three major MAPK pathways such as JNK, p38MAPK and ERK1/2 by using Western blot analysis. We observed that H1 significantly suppressed the expression level of p-ERK1/2 in a dose-dependent manner in KBv200 cells, but the expression level of JNK and p38MAPK was not changed (data not shown). Moreover, H1 also inhibited upstream signaling molecular of ERK1/2, such as p-MEK1/2 (Fig. 5). Subsequently knockdown ERK1 and ERK2 genes by using RNA interference, we observed that Pgp expression was decreased in KBv200 cells (Fig 5b). In addition, we also found that PD98059 and U0126 inhibited Pgp expression. Based on these findings, it suggests that H1 suppressed Pgp expression may be involve a blockade of the MEK-ERK pathway.
In conclusion, our current study demonstrates that H1, a novel derivative of tetrandrine, is efficacious in reversing Pgp-mediated MDR by inhibiting transport function and expression of Pgp. In addition, the reversal of MDR by H1 is associated with inhibition of Vrp-stimulated ATPase activity, an increase of the ubiquitination of Pgp and the blockade of MEK-ERK pathway. These findings demonstrated that H1 is effectively and potential agent in reversing Pgp-mediated MDR.
References
Nobili S, Landini I, Giglioni B, Mini E (2006) Pharmacological strategies for overcoming multidrug resistance. Curr Drug Targets 7:861–879
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234
Staudinger R, Knaus HG, Glossmann H (1991) Positive heterotropic allosteric regulators of dihydropyridine binding increase the Ca2+ affinity of the L-type Ca2+ channel. Stereoselective reversal by the novel Ca2+ antagonist BM 20.1140. J Biol Chem 266:10787–10795
Fu L, Liang Y, Deng L, Ding Y, Chen L, Ye Y, Yang X, Pan Q (2004) Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol 53:349–356
Fu LW, Zhang YM, Liang YJ, Yang XP, Pan QC (2002) The multidrug resistance of tumour cells was reversed by tetrandrine in vitro and in xenografts derived from human breast adenocarcinoma MCF-7/adr cells. Eur J Cancer 38:418–426
Jin J, Wang FP, Wei H, Liu G (2005) Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotetrandrine. Cancer Chemother Pharmacol 55:179–188
Chen LM, Liang YJ, Zhang X, Su XD, Dai CL, Wang FP, Yan YY, Tao LY, Fu LW (2009) Reversal of Pgp-mediated multidrug resistance by Bromotetrandrine in vivo is associated with enhanced accumulation of chemotherapeutical drug in tumor tissue. Anticancer Res 29:4597–4604
Zhang XH, Zhang FY, Ji XJ, Li ZY (1994) Vincristine-resistant human KB cell line and mechanism of multidrug resistance. Yao Xue Xue Bao 29:246–251
Ullmann CA, Eliot HM, Sinha BK (1991) Distribution, DNA damage and cytotoxic effects of etoposide in human tumor xenografts in vivo. Anticancer Res 11:1379–1382
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Huang M, Liu G (1999) The study of innate drug resistance of human hepatocellular carcinoma Bel7402 cell line. Cancer Lett 135:97–105
Zheng LS, Wang F, Li YH, Zhang X, Chen LM, Liang YJ, Dai CL, Yan YY, Tao LY, Mi YJ, Yang AK, To KK, Fu LW (2009) Vandetanib (Zactima, ZD6474) antagonizes ABCC1- and ABCG2-mediated multidrug resistance by inhibition of their transport function. PLoS One 4:e5172
Mistry P, Stewart AJ, Dangerfield W, Okiji S, Liddle C, Bootle D, Plumb JA, Templeton D, Charlton P (2001) In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res 61:749–758
Zhang Z, Wu JY, Hait WN, Yang JM (2004) Regulation of the stability of P-glycoprotein by ubiquitination. Mol Pharmacol 66:395–403
Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y (2007) Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther 6:2092–2102
Dano K (1973) Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta 323:466–483
Modok S, Mellor HR, Callaghan R (2006) Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr Opin Pharmacol 6:350–354
Mimeault M, Hauke R, Batra SK (2008) Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin Pharmacol Ther 83:673–691
Kim H, Barroso M, Samanta R, Greenberger L, Sztul E (1997) Experimentally induced changes in the endocytic traffic of P-glycoprotein alter drug resistance of cancer cells. Am J Physiol 273:C687–C702
McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D’Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M (2006) Roles of the RAF/MEK/ERK and PI3 K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 46:249–279
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773:1263–1284
Zhou J, Liu M, Aneja R, Chandra R, Lage H, Joshi HC (2006) Reversal of P-glycoprotein-mediated multidrug resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer Res 66:445–452
Barancik M, Bohacova V, Kvackajova J, Hudecova S, Krizanova O, Breier A (2001) SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur J Pharm Sci 14:29–36
Acknowledgments
This work was supported by Grants (No. 30630069) from China National Natural Sciences Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, N., Sun, H., Wang, F. et al. H1, a novel derivative of tetrandrine reverse P-glycoprotein-mediated multidrug resistance by inhibiting transport function and expression of P-glycoprotein. Cancer Chemother Pharmacol 67, 1017–1025 (2011). https://doi.org/10.1007/s00280-010-1397-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1397-7