Abstract
Purpose
The rate of hypersensitivity reactions in patients receiving carboplatin (CBDCA) has been reported to increase after multiple doses of the agent. However, risk factors for these onsets have not been well described. In this study, we investigated the contribution of the reported risk factors to the onset of CBDCA-related delayed hypersensitivity reactions.
Methods
We reviewed the records of gynecologic cancer patients receiving CBDCA of more than 7 cycles in Mie University Hospital from March 2006 to July 2009. The patients were divided into two groups on the basis of whether hypersensitivity reactions developed (13 patients) or not (43 patients). Thereafter, the potential influences of the patients’ characteristics on the development of CBDCA-related delayed hypersensitivity reactions were explored using logistic regression analyses.
Results
The median CBDCA-free interval (10 months) in patients with hypersensitivity reactions was significantly higher than that (3 months) in patients without hypersensitivity reactions. Logistic regression analyses revealed a CBDCA-free interval >13 months (odds ratio 22.2, 95% confidence interval 2.57–192, p < 0.01) and a maximum dose of CBDCA > 650 mg (odds ratio 9.52, 95% confidence interval 1.04–93.9; p < 0.05) were significantly correlated with the incidence of CBDCA-related delayed hypersensitivity reactions.
Conclusions
Careful attention should be paid to the onset of delayed hypersensitivity reactions for recurrent gynecologic cancer patients receiving CBDCA > 650 mg after an interval of more than 13 months from the previous CBDCA administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carboplatin (CBDCA) is one of the most commonly used and well-tolerated antineoplastic drugs for gynecologic cancers. A particular appeal of CBDCA is its low risk of nephrotoxicity and neurotoxicity, as well as the relatively low associated incidence of severe emesis, particularly in comparison to cisplatin. It is considered a standard first-line regimen with paclitaxel for ovarian cancers, in addition to known activity against both endometrial and cervical cancers. However, as a result of its extended use, an increased incidence in CBDCA-related hypersensitivity reactions has been widely observed [1–3]. The rate of hypersensitivity reactions in patients receiving more than 7 cycles of CBDCA has been reported to be 26.7%, although it is less frequent (12.5%) during the initial regimen of CBDCA [1]. These reactions can be either mild cutaneous eruptions or more serious anaphylactic symptoms such as hypotension, bronchospasm, and cardiovascular collapse.
It has been reported that a CBDCA-free interval greater than 12 months could be associated with an increased risk of CBDCA-related hypersensitivity reactions [2]. In addition, these incidences in ovarian cancer patients (7.9%) were reported to be significantly higher than those in the overall cancer patient population (2.6%) in the USA [3]. Other reported factors associated with CBDCA-related delayed hypersensitivity reactions are positive history of prior drug allergies, previous CBDCA exposure, and cumulative doses of CBDCA [3–5]. However, to date, the contribution of these factors to the onset of CBDCA-related delayed hypersensitivity reactions has not been fully understood.
In this study, we analyzed the incidence of CBDCA-related delayed hypersensitivity reactions in gynecologic cancer patients receiving CBDCA of more than 7 cycles to investigate the risk factors for their onset.
Methods
Subjects
This study was conducted from March 2006 to July 2009. The three pharmacists in the study group reviewed the medical records (physician, nurse and pharmacist records) of all patients who admitted in Mie University Hospital and received intravenous CBDCA more than 7 cycles for the treatment of gynecologic cancers within the selected time period. All subjects (n = 56) were inpatients at the Obstetrics and Gynecology ward of the Hospital. The median age was 60 years old. Types of cancers were as follows: ovarian cancer (n = 35), endometrial cancer (n = 15), cervical cancer (n = 4), vaginal cancer (n = 1) and ovarian duct cancer (n = 1). The median number of CBDCA administrations was 15 cycles. All patients enrolled in this study had a history of concomitant use of taxanes and CBDCA in the past. Seventeen patients had a history of drug or food allergy. This study was reviewed and approved by the Ethics Committee of Mie University.
Investigation of the risk factors for CBDCA-related delayed hypersensitivity reactions
To investigate the risk factors for CBDCA-related delayed hypersensitivity reactions, patients who received CBDCA more than 7 cycles were divided into two groups on the basis of whether hypersensitivity reactions had developed (13 patients) or not (43 patients). The diagnosis of onset of hypersensitivity reactions was assessed by typical allergy symptoms, red flares, pruritus, respiratory discomfort, reduced blood pressure, shock, and pharynx complaints, etc. We compared the age, type of cancer, number of CBDCA administrations, maximum dose/body of CBDCA after 7 cycles of CBDCA-based regimens, cumulative dose of CBDCA, CBDCA-free interval and histories of drug or food allergy between the two groups. We also explored the potential influences of the patients’ characteristics on the development of CBDCA-related delayed hypersensitivity reactions by logistic regression analyses.
Statistical analysis
The Kolmogorov–Smirnov test was performed to determine whether metric variables used in this study were in normal distribution or not. The Student’s t test or Aspin-Welch test was used for the normal distributed metric variables to assess differences between patients with and without CBDCA-related hypersensitivity reactions. As for the metric variables which were not in normal distribution, Wilcoxon’s rank sum test was used. The Fisher’s exact test or chi-square test was used for categorical types of data to assess differences between the two groups. We calculated the area under receiver-operator characteristics (ROC) curves to estimate the sensitivity, specificity, accuracy and cut off values for some metric variables which did not show normal distribution by the Kolmogorov–Smirnov test. The correlation between the patients’ characteristics investigated and the development of hypersensitivity reactions was analyzed using univariate and multivariate logistic regression analyses. All analyses were done using SAS software (version 9.1.3; SAS Institute Japan Ltd, Tokyo, Japan). Regarding the recorded p values obtained by the Kolmogorov–Smirnov test, values of less than 0.10 were considered statistically significant. Other recorded p values were two sided and values of less than 0.05 were considered statistically significant.
Results
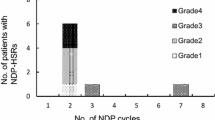
In this study, 13 out of 56 patients developed CBDCA-related hypersensitivity reactions after more than 7 cycles of CBDCA-based regimens. Table 1 shows these patients’ characteristics when CBDCA-related hypersensitivity reactions firstly occurred. The symptoms of hypersensitivity reactions were red flares, pruritus, respiratory discomfort, reduced blood pressure, hives, shock, pharynx complaints, malaise, and facial flush. There were 4 patients with severe hypersensitivity reactions, i.e., respiratory discomfort, reduced blood pressure, and shock, and all of them received CBDCA to treat recurrent gynecologic cancers and had a CBDCA-free interval ≥ 18 months. Only one patient received cisplatin and radiation therapy and no patient received anthracycline antitumor drugs before CBDCA-related hypersensitivity reactions.
In Table 2, demographic characteristics were compared between the patients with and without CBDCA-related hypersensitivity reactions in this study. The Kolmogorov–Smirnov test revealed that the maximum dose of CBDCA (p < 0.01) and CBDCA-free interval (p < 0.01) did not show normal distribution among the metric variables used in this study. There was a significant difference between the two groups regarding the CBDCA-free interval (Wilcoxon rank sum test, p < 0.05). No significant differences were found between the two groups with regard to the other factors investigated.
As for the two metric variables, the maximum dose/body of CBDCA and CBDCA-free interval which did not show normal distribution, we performed ROC curve analyses. The results showed that adequate cut off values for the maximum dose/body of CBDCA after 7 cycles of CBDCA based regimens and CBDCA-free interval were 650 mg and 13 months, respectively. When using these cut off values, accuracy (sensitivity, specificity) of the maximum dose/body and CBDCA-free interval to detect the incidence of CBDCA-related hypersensitivity reactions were 69.6% (38.5, 79.1%) and 82.1% (46.2, 93.0%), respectively.
Table 3 shows the results of univariate logistic regression analysis regarding the potential influences of the patients’ characteristics on the development of CBDCA-related hypersensitivity reactions. The only factor that significantly contributed to the onset of CBDCA-related hypersensitivity reactions was CBDCA-free interval > 13 months (odds ratio 6.51, 95% confidence interval 1.55–27.4; p < 0.05). The results of the multivariate logistic regression analysis to determine the risk factors contributing to the development of CBDCA-related hypersensitivity reactions are shown in Table 4. This method allows regression analysis to estimate the influence of covariates on a clinical outcome. The multivariate analysis revealed that a CBDCA-free interval > 13 months (odds ratio 22.2, 95% confidence interval 2.57–192; p < 0.01) and maximum CBDCA dose/body > 650 mg after 7 cycles of CBDCA-based regimen (odds ratio 9.52, 95% confidence interval 1.04–93.9; p < 0.05) were independent risk factors significantly contributing to the incidence of CBDCA-related hypersensitivity reactions.
Discussion
Over the past decade, CBDCA use has increased for both initial and recurrent treatment of gynecologic cancers because of its proven high efficacy and tolerability. However, one common concern is the risk of hypersensitivity reactions, which are known to be more common at retreatment. In the present study, 23.2% (13 out of 56 patients) of patients developed CBDCA-related hypersensitivity reactions after 7 cycles of CBDCA-based regimens. This rate was close to a previous report showing the rate of hypersensitivity reactions in patients receiving more than 7 cycles of CBDCA was 26.7% (20 out of 75 patients) [1]. Some researchers have reported that a CBDCA-free interval longer than 12 months, ovarian cancer, and positive history of prior drug allergies and/or CBDCA exposure contributed to the incidence of CBDCA-related hypersensitivity reactions [2, 3]. Moreover, Koshiba et al. recently reported that the rate of severe CBDCA-related hypersensitivity reactions greatly increased beyond 15 cycles and/or cumulative doses of 8,000 mg in Japanese women [4].
Different from previous studies investigating the risk factors for hypersensitive reactions to CBDCA, we conducted this study by restricting the patients those receiving CBDCA for more than 7 cycles because patients receiving CBDCA for 7 or less cycles might develop hypersensitivity reactions with further CBDCA administrations. Moreover, these study subjects could provide characteristics of patients who have a risk of delayed hypersensitivity reactions by CBDCA. Based on our study, a CBDCA-free interval > 13 months and a maximum dose/body of CBDCA > 650 mg were independent risk factors for the incidence of CBDCA-related delayed hypersensitivity reactions whereas type of cancer, number of CBDCA administrations, cumulative dose of CBDCA and positive history of prior drug allergies did not seem to be correlated with these events. Multivariate logistic regression analyses indicated the patients with a CBDCA-free interval > 13 months had about a 22-fold higher risk of developing hypersensitivity onset in gynecologic cancer patients. In particular, all four patients with severe hypersensitivity reactions, i.e., respiratory discomfort, reduced blood pressure, and shock received CBDCA to treat recurrent gynecologic cancers and had a CBDCA-free interval ≥ 18 months.
The present study is thought to give the first statistical evidence that the maximum dose/body after 7 cycles of CBDCA-based regimens was significantly correlated with the onset of delayed hypersensitivity reactions due to CBDCA. Multivariate logistic regression analysis revealed a CBDCA dose/body > 650 mg after 7 cycles of CBDCA-based regimens had a 9.5-fold increased risk of hypersensitivity onset. It was reported that the mean dose/cycle of CBDCA at which the first hypersensitivity reaction occurred in patients (614.8 mg) with hypersensitive reactions was significantly higher than that in patients (500.0 mg) without these events [4]. The exact mechanism of the development of anticancer-related hypersensitivity has not been clarified, but is thought to be mainly due to type I hypersensitivity, immunoglobulin E (IgE)-mediated, or due to histamine release [6–9]. l-Asparaginase, etoposide, dacarbazine, and fluorouracil-related hypersensitivities have been considered to be related to high dose and/or short-term infusions [7]. In the case of CBDCA, hypersensitivity reactions of refinery workers to platinum salts have been carefully described, and the mechanism of platinum hypersensitivity in these workers is thought to be an IgE-mediated reaction with low molecular weight platinum salts acting as a haptenic compound [10]. It was reported that there was an association with a reduced incidence of hypersensitivity reactions of CBDCA with an extended infusion schedule [11]. In addition, the platinum agent oxaliplatin was reported to be re-administrated safely after hypersensitivity reactions by using 10-fold dilutions in a sufficient volume to administer the total dose or by slowing the infusion rate [12]. These reports show some agreement with our results that CBDCA-related type-I hypersensitivity reactions occurred at a higher rate in the patients receiving higher doses (>650 mg) of CBDCA infusion. However, despite low dose CBDCA, five cases (21.7%) of hypersensitivity reactions were reported in patients with recurrent ovarian cancers receiving low-dose paclitaxel (60 mg/m2) and CBDCA (AUC = 2) [13]. In our study, although 16 patients received low-dose/body CBDCA (≤300 mg) after 7 cycles of the drug, only one developed a mild hypersensitivity reaction (pharynx complaint), which did not require discontinuation of CBDCA administration.
This study shows that a CBDCA-free interval > 13 months and a CBDCA dose/body > 650 mg after 7 cycles of CBDCA-based regimens were possible independent risk factors significantly contributing to the development of CBDCA-related hypersensitivity reactions. Patients with gynecologic cancers often receive prolonged CBDCA therapies as neoadjuvant/adjuvant chemotherapies and treatment for recurrent cancers. Therefore, careful attention should be paid to the onset of delayed hypersensitivity reactions for recurrent gynecologic cancer patients receiving CBDCA > 650 mg/body after an interval of > 13 months from previous CBDCA administration. These findings provide useful information for health care workers regarding the prediction of CBDCA-related delayed hypersensitivity reactions, aiding the safe administration of CBDCA.
References
Markman M, Kennedy A, Webster K, Elson P, Peterson G, Kulp B, Belinson J (1999) Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol 17:1141–1145
Schwartz JR, Bandera C, Bradley A, Brard L, Legare R, Granai CO, Dizon DS (2007) Does the platinum-free interval predict the incidence or severity of hypersensitivity reactions to carboplatin? The experience from Women and Infants’ Hospital. Gynecol Oncol 105:81–83
Navo M, Kunthur A, Badell ML, Coffer LW 2nd, Markman M, Brown J, Smith JA (2006) Evaluation of the incidence of carboplatin hypersensitivity reactions in cancer patients. Gynecol Oncol 103:608–613
Koshiba H, Hosokawa K, Kubo A, Miyagi Y, Oda T, Miyagi Y, Watanabe A, Honjo H (2009) Incidence of Carboplatin-related hypersensitivity reactions in Japanese patients with gynecologic malignancies. Int J Gynecol Cancer 19:460–465
Weidmann B, Mülleneisen N, Bojko P, Niederle N (1994) Hypersensitivity reactions to carboplatin. Report of two patients, review of the literature, and discussion of diagnostic procedures and management. Cancer 73:2218–2222
Weiss RB (1992) Hypersensitivity reactions. Semin Oncol 19:458–477
Shepherd GM (2003) Hypersensitivity reactions to chemotherapeutic drugs. Clin Rev Allergy Immunol 24:253–262
Sliesoraitis S, Chikhale PJ (2005) Carboplatin hypersensitivity. Int J Gynecol Cancer 15:13–18
Zanotti KM, Rybicki LA, Kennedy AW, Belinson JL, Webster KD, Kulp B, Peterson G, Markman M (2001) Carboplatin skin testing: a skin-testing protocol for predicting hypersensitivity to carboplatin chemotherapy. J Clin Oncol 19:3126–3129
Cromwell O, Pepys J, Parish WE, Hughes EG (1979) Specific IgE antibodies to platinum salts in sensitized workers. Clin Allergy 9:109–117
O’Cearbhaill R, Zhou Q, Iasonos A, Hensley ML, Tew WP, Aghajanian C, Spriggs DR, Lichtman SM, Sabbatini PJ (2010) The prophylactic conversion to an extended infusion schedule and use of premedication to prevent hypersensitivity reactions in ovarian cancer patients during carboplatin retreatment. Gynecol Oncol (in press)
Syrigou EI, Karapanagiotou EM, Alamara CV, Boura PG, Saif MW, Syrigos KN (2009) Hypersensitivity reactions to oxaliplatin: a retrospective study and the development of a desensitization protocol. Clin Colorectal Cancer 8:106–109
Watanabe Y, Nakai H, Ueda H, Nozaki K, Hoshiai H (2005) Carboplatin hypersensitivity induced by low-dose paclitaxel/carboplatin in multiple platinum-treated patients with recurrent ovarian cancer. Int J Gynecol Cancer 15:224–227
Conflict of interest statement
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugimoto, H., Iwamoto, T., Murashima, Y. et al. Risk factors contributing to the development of carboplatin-related delayed hypersensitivity reactions in Japanese patients with gynecologic cancers. Cancer Chemother Pharmacol 67, 415–419 (2011). https://doi.org/10.1007/s00280-010-1338-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1338-5