Abstract
The anti-diabetic thiazolidinedione compound pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, and selective cyclooxygenase-2 inhibitors are clinically used in patients with advanced malignancies. Several previously published in vivo and in vitro studies showed growth inhibitory effects on different cancer cell lines. However, the underlying mechanisms are fairly unclear. Here, we analyzed the effects of pioglitazone in combination with other drugs in a three-dimensional multicellular tumor spheroid culture system (MCTS) generated from the two prostate carcinoma cell lines PC3 and LNCaP. As expected, pioglitazone also inhibited tumor cell proliferation in the MCTS system. Further studies revealed that pioglitazone lowered the pH of the culture medium, decreased oxygen consumption and increased lactate secretion in both tumor cell lines. Other glitazones, troglitazone and ciglitazone, had similar effects. The combination of pioglitazone with 2-deoxyglucose, a potent inhibitor of glycolysis, had an additive effect on the inhibition of cell proliferation and led to MCTS disintegration. Our data propose a new mechanism of growth inhibition by pioglitazone through modulation of the tumor cell metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glitazones and non-steroidal anti-inflammatory drugs (NSAIDs) have been reported to exert anti-neoplastic effects on different tumor entities in vitro and in vivo involving induction of differentiation, apoptosis and cell cycle arrest as well as inhibition of angiogenesis and metastasis [1].
Pioglitazone belongs to the class of glitazones or thiazolidinediones, a series of oral anti-diabetic drugs. Pioglitazone acts as a ligand for peroxisome proliferator-activated receptor γ (PPARγ), which serves as a transcription factor belonging to the nuclear receptor superfamily. After heterodimerization with the retinoid X receptor, PPARγ binds to PPARγ-response elements (PPRE) [2] and regulates target genes involved in adipocyte differentiation, lipid storage and glucose homeostasis [3]. In type II diabetes mellitus, it exerts glucose-lowering effects via increasing glucose uptake and metabolism [4]. These changes in the metabolic activity are linked to the induction of numerous PPARγ-regulated genes such as glucose transporters Glut-2 and Glut-4 [3, 5, 6].
Several effects of glitazones may contribute to their anti-neoplastic activity. Inhibition of proliferation by PPARγ-agonists has been shown for many tumor cell lines [1] but did not always correlate with PPARγ-receptor expression [7]. In addition, pro-apoptotic effects such as the decrease of bcl2 or increase in caspase activation have been described [8–10]. However, anti-proliferative effects of glitazones vary depending on the compound and cell line used [9, 10]. Ohta et al. [11] found condensation of the nucleus and chromatin fragmentation in thyroid carcinoma cells treated with troglitazone but no effect on blc-2 or bax expression. More recently, the anti-proliferative effect of glitazones could also be demonstrated in primary cultures generated from thyroid carcinoma [12], whereas normal human thyroid follicular cells were not affected by the drug [13].
Warburg proposed many years ago that tumor cells mainly depend on glucose metabolism and are characterized by an accelerated glycolysis [14]. In the meantime, it is known that hypoxia or oncogenic transformation leads to the upregulation of glycolytic enzymes such as lactate dehydrogenase (LDH) and thereby induce this “glycolytic switch” [15, 16]. Furthermore, tumor cell metabolism is altered in many other ways, e.g. an increased expression of cyclooxygenase (COX) has been described for several tumors. While COX-1, an enzyme which is responsible for the formation of prostanoids, is constitutively expressed in almost all tissues, its isoenzyme COX-2 is rapidly induced by certain inflammatory cytokines, growth factors and oncogenes [17]. Based on these findings, much attention is focused on COX-2 as a potential target for tumor therapy. COX-2 inhibitors have been shown to exert anti-proliferative and pro-apoptotic effects in vitro [17]. In addition, COX inhibitors such as indomethacin also target the PPARγ signaling pathway [18]. Based on the anti-neoplastic action of glitazones and COX-2-inhibitors as well as the interrelationship between their pathways, several in vitro and in vivo studies analyzed a possible synergistic effect of both drugs. The proliferation of a breast carcinoma cell line was synergistically inhibited and apoptotic rates were markedly increased by co-incubation of the COX-2 inhibitor NS-398 with ciglitazone [19]. In addition, Sun et al. [20] recently published a synergistic effect of NS-398 and rosiglitazone on proliferation and apoptosis in a pancreas carcinoma cell line. Clinical studies have demonstrated an effect of COX-2 inhibitors and PPARγ agonists in glioma [21] and melanoma [22].
In contrast, the anti-proliferative effect of pioglitazone on prostate carcinoma cell lines in our three-dimensional multicellular tumor spheroid (MCTS) culture system in vitro was not enhanced by the addition of rofecoxib. However, pioglitazone alone decreased the proliferation and altered glucose metabolism as demonstrated by acidification of the culture medium, decreased oxygen consumption and increased lactate secretion. We suggest that the growth inhibitory effect of pioglitazone is at least partially due to its effect on glucose metabolism. The combination of pioglitazone with 2-deoxyglucose, a potent inhibitor of glycolysis, had an additive effect on the inhibition of cell proliferation and led to the disintegration of tumor spheroids. These data suggest that tumor cell metabolism is an important target for tumor therapy.
Materials and methods
Chemicals
Pioglitazone and rofecoxib were kindly provided by Takeda Pharma GmbH, Germany and MSD Sharp & Dohme GmbH, Germany. Ciglitazone and troglitazone were obtained from Sigma. DMSO or ethanol was used in equal molarity as a control. 2-Deoxyglucose (Sigma) was used at a final concentration of 10 mM in medium, rotenone (Sigma) was used at 1 μM.
Cell culture
The prostate carcinoma cell lines LNCaP and PC3 (ATCC) were cultured in RPMI 1640 supplemented with 10% fetal calf serum (both from PAA), 2 mM glutamine, 50 U/ml penicillin/50 μg/ml streptomycin, 0.4% vitamins and 1% non-essential amino acids (all from Gibco) in a humidified 5% CO2 atmosphere at 37°C. Multicellular tumor spheroids (MCTS) were generated by the liquid-overlay technique [23]. In brief, a suspension of subconfluent monolayer-cultured tumor cells was seeded at a density of 5,000 cells/well in agarose-coated 96-well plates. After 4 days, three-dimensional MCTS had formed and were harvested and analyzed.
For light microscopy, spheroids were documented using an imaging processing system consisting of an inverted microscope (AxioVert 200) equipped with a digital camera (AxioCam MRc) and software (KS300) (all from Carl Zeiss, Göttingen, Germany).
Determination of cell proliferation
For proliferation assays, MCTS were generated in 96-well plates as described earlier. After 4 days, half of the medium was exchanged for medium with various drugs and DMSO as control. After incubation for 48 h, [3H]thymidine was added and counts were analyzed after further incubation of 24 h. Spheroid experiments were done in six replicates and repeated at least three times.
For proliferation assays in monolayer culture, cells were seeded in 96-well plates in a density of 3 × 104 cells/well. After 2 h, drugs were added for 48 h. Thereafter, cells were labelled with 0.5 μCi/well [3H]thymidine for 24 h and counts were detected after solubilizing. Each experiment was done in quadruples and repeated at least three times.
Cell cycle analysis
For cell cycle analysis in monolayer culture, PC3 and LNCaP were seeded at a concentration of 2.5 × 106 cells/flaks, and 2 h later, pioglitazone or DMSO as a control was added for 2 days. For analysis in MCTS cultures, preformed spheroids at day 4 were incubated with pioglitazone or DMSO as a control for 48 h.
Thereafter, cells were harvested by mild enzymatic digestion, washed twice in PBS and counted with a Casy 1 cell analyzer system (Schaerfe, Reutlingen, Germany). Cells were fixed in 70% methanol (Merck, Darmstadt, Germany) at 4°C overnight at a concentration of 1 × 106 cells/ml. Methanol-permeabilized cells were stored at 4°C for a maximum of 3–4 weeks. For propidium iodide staining, single cell suspensions were again washed, resuspended in PBS (1 × 106 cells in 425 μl) and pre-incubated for 20 min at 37°C with RNase by adding 50 μl of a 1 mg/ml stock solution (Sigma). Then, propidium iodide was added at a concentration of 50 μg/ml (stock: 1 mg/ml PI, Sigma). After 5 min, cell suspensions were transferred on ice and DNA histograms were recorded using a FACSCalibur flow cytometer. For each sample, 2.5 × 104 cells were recorded. Pulse processing was turned on to record signal height, area and width for adequate doublet exclusion. High-quality cell cycle analysis was performed with the MultiCycle software package (Phoenix Flow Systems, San Diego, CA, USA).
Determination of pH and oxygen consumption
The SDR SensorDish® Reader (PreSens Precision Sensing GmbH, Regensburg, Germany), a small, 24-channel oxygen and pH meter was used to determine pH and oxygen consumption. The corresponding optical sensors for oxygen (OxoDish®) or pH (HydroDish®) are integrated at the bottom of each well of 24-well multidishes (PreSens Precision Sensing GmbH, Regensburg, Germany). The sensors are luminescent dyes embedded in an analyte-sensitive polymer. The luminescence lifetime of these dyes depends on the amount of analyte. The sensors are read out non-invasively through the bottom of the multidish by the SensorDish® Reader. The resulting signal is converted automatically to the respective parameter (dissolved oxygen or pH) using calibration parameters stored in the software. The SensorDish® Reader was used in the incubator for the whole duration of the cultivation and measurements were performed in user-defined intervals.
For the determination of pH and oxygen consumption of multicellular tumor spheroid cultures, spheroids were generated as described previously and harvested. Nineteen spheroids, corresponding to about 2 × 105 cells, were given into one well in 1 ml medium and analyzed as described earlier.
Determination of lactate in tumor cell supernatants
For lactate determination in MCTS supernatants, PC3 and LNCaP MCTS were set up. At day 4, medium was changed and cells were incubated for additional 2 days in the presence of pioglitazone. For lactate determination in monolayer culture, cells were seeded with and without pioglitazone, troglitazone or ciglitazone. Lactate in the supernatant was measured after 48 h. The analysis was performed by a routine procedure with a ADVIA 1650 (Bayer, Terrytown, NY, USA) and specific reagents (Department of Clinical Chemistry, University of Regensburg). Given is the mean of at least three independent experiments, ±SEM.
Results
Pioglitazone inhibits tumor cell proliferation
Several in vitro studies described growth inhibiting effects of the PPARγ agonist pioglitazone and COX-2 inhibitors on tumor cells. In contrast to these investigations in monolayer cultures, we analyzed drug effects in multicellular tumor spheroid (MCTS) cultures. This three-dimensional cell culture model better mimics the in vivo situation found in a tumor with respect to growth kinetics, extracellular matrix production, nutrient gradients, oxygen tension and pH [24, 25]. PC3 and LNCaP, both prostate carcinoma cell lines, were seeded for MCTS generation. We have chosen these two cell lines because they show differences in the expression of PPARγ. Whereas PC3 exhibits a strong expression of PPARγ, PPARγ levels are very low in LNCaP [26]. After 4 days, the preformed MCTS were incubated for additional 2 days with pioglitazone and/or rofecoxib and ³[H] thymidine incorporation was determined after additional 24 h. Pioglitazone alone as well as in combination with rofecoxib significantly inhibited the proliferation of PC3 and LNCaP MCTS indicating that the effect is at least partially PPARγ-independent. Rofecoxib alone had no effect on tumor cell proliferation (Fig. 1a, b).
Inhibition of cell proliferation in PC3 and LNCaP multicellular tumor spheroid cultures. PC3 (a) and LNCaP (b) multicellular tumor spheroids (MCTS) were generated for 4 days and then incubated for additional 2 days in the presence of pioglitazone (pio, 50 μM), rofecoxib (rofe, 20 μM), a combination of both drugs (pio+rofe) or DMSO (control). ³[H] thymidine was added, and its incorporation was determined after additional 24 h. Given is the mean ± SEM of five independent experiments, *P < 0.02. c Cell cycle inhibition by pioglitazone was analyzed in PC3 and LNCaP. Preformed MCTS at day 4 were treated with pioglitazone (pio, 50 μM) or DMSO (control) and incubated for 48 h. After harvesting and permeabilization, cells were treated with propidium iodide and the percentage of cells in S-phase was recorded using a FACSCalibur flow cytometer. Given is the mean ± SEM of at least three independent experiments
Apoptosis induction is one possible reason for the growth inhibitory effect of pioglitazone. Therefore, we analyzed PC3 and LNCaP cultures by Annexin-V/PI staining after incubation with pioglitazone. When compared to camptothecin, a known inducer of apoptosis in prostate carcinoma cells [27], we could not find a significant induction of cell death in the presence of pioglitazone or rofecoxib (data not shown).
To further investigate the growth inhibitory effect, cell cycle analyses were performed in MCTS cultures. As shown in Fig. 1c, pioglitazone led to a significant reduction in the number of cells in S-phase in PC3, whereas in LNCaP MCTS, no significant reduction was found. Rofecoxib again did not exert an inhibitory effect on PC3 and LNCaP spheroid cultures (data not shown). As rofecoxib did not show an inhibitory effect in cell proliferation assays in our MCTS cultures, we omitted this drug in further experiments.
Pioglitazone shifts glucose metabolism to glycolysis
Interestingly, during the incubation of several cell lines with pioglitazone, we noted a strong acidification of the culture medium when compared to control cultures. We started to analyze this acidification in detail using the SDR SensorDish® Reader (PreSens Precision Sensing GmbH, Regensburg, Germany), a small, 24-channel pH meter with luminescent dyes as sensors. Medium pH was continuously determined every 30 s over a period of 24 h in MCTS cultures in the absence or presence of pioglitazone. During this 24-h incubation period, we determined a slight decrease in the medium pH in the control cultures. The addition of pioglitazone accelerated acidification of the PC3 culture medium, and differences were detectable after about 10 h (Fig. 2a).
Acidification of the culture medium and oxygen consumption in tumor cells after incubation with pioglitazone. a The pH of the cell culture medium was recorded by the SDR SensorDish® Reader. PC3 and LNCaP MCTS were generated as described before, harvested after 4 days, and 19 spheroids (corresponding to about 2 × 105 cells) were given into one well of the SensorDish® plate. Pioglitazone (50 μM) or DMSO as a control was added, and the pH was recorded over 24 h. One representative example is given for PC3 and LNCaP comparing control and pioglitazone treatment. b The oxygen consumption in PC3 and LNCaP MCTS cultures was recorded with the SDR SensorDish® Reader (PreSens Precision Sensing GmbH, Regensburg, Germany) using the same experimental settings as described in (a)
As acidification is most likely linked to a shift from respiration to glycolysis, we analyzed the oxygen consumption of MCTS cultures in the SDR SensorDish® Reader using oxygen sensor dishes (PreSens Precision Sensing GmbH, Regensburg, Germany). The oxygen concentration in the culture medium was again recorded every 30 s for 24 h. The oxygen concentration in the culture medium is representative for the oxygen consumption of the cells. As shown in Fig. 2b, the oxygen consumption was strongly decreased in the presence of pioglitazone when compared to the control. The effect was already detectable after 2–4 h in PC3 cultures indicating that inhibition of cell proliferation is not responsible for this effect (Fig. 2b). To analyze the effects of pioglitazone on non-cancer cells, we measured the oxygen consumption of primary human monocytes. Monocytes showed a significant oxygen consumption, however, pioglitazone had only a marginal effect on oxygen consumption indicating that the effect is specific for tumor cells (data not shown).
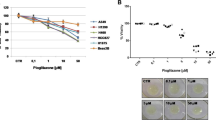
Once oxygen consumption is reduced and glucose metabolism is shifted to glycolysis, increased amounts of lactate, the end product of glycolysis, should be secreted into the culture medium. In line with this assumption, pioglitazone significantly increased lactate secretion in PC3 MCTS and monolayer cultures, whereas in LNCaP, a significant increase was only found in monolayer cultures (Fig. 3a, b). As cell proliferation was inhibited by pioglitazone, it has to be taken into account that in the presence of pioglitazone, less cells secreted even higher amounts of lactate. Furthermore, the weaker effects that we detected in LNCaP may in part be due to lower expression levels of PPARγ. Similar effects on lactate production were obtained for two other glitazones, ciglitazone and troglitazone. The effects were dose-dependent for pioglitazone and ciglitazone, whereas troglitazone showed a maximal response already at 5 μM (Fig. 3c). In contrast, when we analyzed oxygen consumption of the three different compounds, all glitazones showed a dose-dependent effect with nearly complete inhibition of oxygen consumption at 50 μM (Fig. 3d).
Pioglitazone increases lactate secretion. a PC3 and LNCaP MCTS were generated for 4 days and then incubated for additional 2 days in the presence of pioglitazone. b PC3 and LNCaP monolayer cultures were set up at a concentration of 2.5 × 106 cells/well with and without pioglitazone (50 μM). c Furthermore, we analyzed the effect of three different glitazones (pioglitazone, troglitazone, ciglitazone) in a concentration range between 5 and 50 μM on PC3 monolayer cultures. All supernatants were harvested after 48-h incubation for lactate determination. Given is the mean of at least three independent experiments, ±SEM. PC3 MCS: **P = 0.0075, PC3 ML: *P = 0.0322, LNCaP MCS: ns, LNCaP ML: *P = 0.0212. d To analyze the effect of different glitazones on oxygen consumption, PC3 monolayer cultures were treated with different concentrations of pioglitazone, troglitazone and ciglitazone. The oxygen consumption was recorded over a period of 24 h with the SDR SensorDish® Reader (PreSens Precision Sensing GmbH, Regensburg, Germany)
Our data suggest that glitazones shifts glucose metabolism from respiration to glycolysis which may be the reason for the decreased cell proliferation. Therefore, we analyzed proliferation and oxygen consumption in the presence of rotenone, an inhibitor of oxidative phosphorylation that interferes with the electron transport chain in mitochondria. As shown in Fig. 4, rotenone completely suppressed oxygen consumption in both cell lines and strongly inhibited cell proliferation (Fig. 4a, b).
Suppression of oxygen consumption and proliferation by rotenone. a To determine the effect of rotenone on oxygen consumption, PC3 and LNCaP were seeded at a concentration of 2 × 105 cells/well into SensorDish® plates with or without 1 μM rotenone. Oxygen concentration in the culture was recorded over 24 h by the SDR SensorDish® Reader. One representative example is given for PC3 and LNCaP. b For proliferation analysis, PC3 and LNCaP were seeded at a concentration of 3 × 104/well with or without 1 μM rotenone. After 48 h, ³[H] thymidine was added and its incorporation determined after additional 24 h. Given is the mean of three independent experiments, ±SD
Pioglitazone alone partially blocked respiration but never reached the inhibitory capacity of rotenone. Therefore, we tried to improve the anti-tumor effect of pioglitazone by the addition of 2-deoxyglucose (2-DG), an inhibitor of hexokinase, a key enzyme in glycolysis [28]. 2-DG alone significantly inhibited proliferation in PC3, but the inhibition was not significant for LNCaP. However, the combination of both pioglitazone and 2-DG showed a strong significant effect not only on PC3, but also on LNCaP (Fig. 5).
Additive effect of 2-deoxyglucose and pioglitazone on cell proliferation. PC3 and LNCaP MCTS were generated for 4 days and then incubated for additional 2 days in the presence of 2-deoxyglucose (10 mM, 2-DG), pioglitazone (50 μM, pio), a combination of pioglitazone and 2-DG (pio+2-DG) or DMSO as a control. ³[H] thymidine was added and its incorporation determined after additional 24 h. Given is the mean of three independent experiments, ±SD
Next, we analyzed morphological changes of MCTS after the addition of pioglitazone, 2-DG or both. Similar to our experimental setting used for proliferation assays, we used pre-established MCTS cultures. On day 4 after seeding, MCTS had formed in the culture and drugs were added for another 2 days.
As shown in Fig. 6, each cell type formed MCTS of a typical morphology. The incubation with pioglitazone, 2-DG or both led to morphological changes. A clearly visible disintegration of spheroids was detected after incubation of PC3 MCTS with pioglitazone in combination with 2-DG. In LNCaP, the size of MCTS was clearly diminished (Fig. 6). Furthermore, when the drugs were added during the initial generation of MCTS, only fuzzy spheroids were formed (data not shown).
Pioglitazone in combination with 2-deoxyglucose leads to disintegration of MCTS. PC3 and LNCaP MCTS were generated and at day 4 incubated with pioglitazone (50 μM), 2-deoxyglucose (10 mM, 2-DG), a combination of both drugs (pio+2-DG), or DMSO as a control. After 48 h, photographs were taken at a magnification of ×50 (PC3) and ×100 (LNCaP)
Discussion
Glitazones, so-called peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists, have been reported to exert anti-neoplastic effects on different tumor entities in vitro and in vivo [29, 30]. However, the mechanisms underlying these anti-neoplastic effects are still under debate. Induction of differentiation, apoptosis and cell cycle arrest has been described [1], but reports are contradictory and seem to be at least in part cell line dependent [9]. Most of the studies used monolayer cultures. However, the situation in solid tumors is better mimicked by a three-dimensional model, where oxygen and nutrient supply as well as extracellular matrix generation and cell–cell contact are more in vivo-like [24]. A recent publication also demonstrated that glucose metabolism is different in three-dimensional culture systems when compared to monolayer cultures [31]. Therefore, we performed our analyses in the three-dimensional model system of multicellular tumor spheroids (MCTS) generated from the two prostate carcinoma cell lines, LNCaP and PC3. The anti-proliferative effect of pioglitazone could be explained by neither the induction of apoptosis nor the detected slight inhibition of cell cycle. This is in line with Kubota et al. [32] who found a significant inhibition of PC3 proliferation but no significant decrease in the number of tumors cells in S-phase of the cell cycle.
Interestingly, when we incubated tumor cells with pioglitazone, we recorded an acidification of the culture medium and a decreased oxygen consumption in the cell cultures. In addition, we detected an increase in lactate in the supernatants despite a decreased proliferation. These findings indicate a shift of cellular glucose metabolism from oxidative phosphorylation to glycolysis induced by pioglitazone. This hypothesis is strengthened by the finding that glitazones reduce the activity of mitochondrial complex I in tissue homogenates of skeletal muscle in vitro [33]. Furthermore, an increased glucose uptake has been demonstrated in the presence of glitazones in canine kidney cells [34] and astrocytes [35]. These results imply that PPARγ agonists are important regulators of glucose metabolism in different cell types. Therefore, we suggest that the metabolic effects of glitazones may contribute to the anti-proliferative activity in tumor cells. In line with this hypothesis, Artwohl and colleagues have shown that an increased lactate release after incubation with glitazones correlated with an inhibition of microvascular and macrovascular cell proliferation. This effect was mimicked by rotenone, a mitochondrial complex I inhibitor [36]. Accordingly, we found that pioglitazone increased lactate production and inhibited proliferation in prostate carcinoma cell lines. A similar effect was found by the addition of rotenone although it had a much stronger effect on oxygen consumption compared to pioglitazone.
Interestingly, the effect of pioglitazone on oxygen consumption was only found in tumor cell lines but not in primary monocytes. This is in line with findings by Antonelli et al. [13] who demonstrated that only thyroid carcinoma cells but not their normal counterparts were affected by glitazone treatment. One might speculate that the differences in sensitivity of normal primary cells and malignant cells are due to different levels of PPARγ receptor expression.
To enhance the anti-neoplastic activity of pioglitazone, a combination of drugs targeting different metabolic pathways might be beneficial. In a phase II trial, the activity of continuously administered pioglitazone and rofecoxib combined with low-dose chemotherapy was analyzed in patients with high-grade gliomas. A moderate activity was detected, even though clinical responses did not correspond with COX-2 and PPARγ-receptor expression [21]. When we used pioglitazone in combination with rofecoxib in vitro, we did not find a stronger growth inhibiting effect on tumor cell proliferation compared to that of pioglitazone alone indicating that in vivo the combination may act not only on the tumor cell itself, but also on the tumor stroma.
As pioglitazone accelerates glycolysis and supports the so-called “Warburg effect”, targeting this glycolytic pathway simultaneously seems to be promising for tumor therapy. We, therefore, tested a combination of pioglitazone and 2-DG, a known inhibitor of hexokinase and key enzyme in glycolysis. In MCTS, which better represent solid tumors when compared to monolayer cultures, we found a clear disintegration when 2-DG was added in combination with pioglitazone. In a first study by Singh et al. [37], it has been shown that the use of 2-DG in glioblastoma patients is save and no acute toxicity occurred, which makes it feasible for clinical use. Therefore, a combination of drugs that shift cells toward glycolysis and decrease oxygen consumption and drugs that block glucose metabolism seems to be a conceivable approach for the treatment of tumor patients.
Another aspect of low oxygen consumption by tumor cells after incubation with pioglitazone is the possible reoxygenation of the tumor environment. It has been shown recently that NSAIDs decrease oxygen consumption, lead to tumor oxygenation and in turn increase the radiosensitivity of tumors [38]. In the light of these findings, pioglitazone could also be used as a sensitizer for radiotherapy of tumors.
In summary, targeting the tumor metabolism seems to be a promising approach for anti-tumor therapy. Combination therapies of thiazolidinediones with drugs that interfere with glucose metabolism or in combination with radiotherapy represent possible new approaches that should be considered for the treatment of cancer patients.
References
Koeffler HP (2003) Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res 9:1–9
Ijpenberg A, Jeannin E, Wahli W, Desvergne B (1997) Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element. J Biol Chem 272:20108–20117
Lee CH, Olson P, Evans RM (2003) Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144:2201–2207
Hauner H (2002) The mode of action of thiazolidinediones. Diabetes Metab Res Rev 18(Suppl 2):S10–S15
Kim HS, Noh JH, Hong SH, Hwang YC, Yang TY, Lee MS, Kim KW, Lee MK (2008) Rosiglitazone stimulates the release and synthesis of insulin by enhancing GLUT-2, glucokinase and BETA2/NeuroD expression. Biochem Biophys Res Commun 367:623–629
Jensterle M, Janez A, Mlinar B, Marc J, Prezelj J, Pfeifer M (2008) Impact of metformin and rosiglitazone treatment on glucose transporter 4 mRNA expression in women with polycystic ovary syndrome. Eur J Endocrinol 158:793–801
Freudlsperger C, Moll I, Schumacher U, Thies A (2006) Anti-proliferative effect of peroxisome proliferator-activated receptor gamma agonists on human malignant melanoma cells in vitro. Anticancer Drugs 17:325–332
Wu JS, Lin TN, Wu KK (2009) Rosiglitazone and PPAR-gamma overexpression protect mitochondrial membrane potential and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family proteins. J Cell Physiol 220:58–71
Shiau CW, Yang CC, Kulp SK, Chen KF, Chen CS, Huang JW, Chen CS (2005) Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer Res 65:1561–1569
Chaffer CL, Thomas DM, Thompson EW, Williams ED (2006) PPARgamma-independent induction of growth arrest and apoptosis in prostate and bladder carcinoma. BMC Cancer 6:53
Ohta K, Endo T, Haraguchi K, Hershman JM, Onaya T (2001) Ligands for peroxisome proliferator-activated receptor gamma inhibit growth and induce apoptosis of human papillary thyroid carcinoma cells. J Clin Endocrinol Metab 86:2170–2177
Antonelli A, Ferrari SM, Fallahi P, Frascerra S, Piaggi S, Gelmini S, Lupi C, Minuto M, Berti P, Benvenga S, Basolo F, Orlando C, Miccoli P (2009) Dysregulation of secretion of CXC alpha-chemokine CXCL10 in papillary thyroid cancer: modulation by peroxisome proliferator-activated receptor-gamma agonists. Endocr Relat Cancer 16:1299–1311
Antonelli A, Ferrari SM, Fallahi P, Berti P, Materazzi G, Minuto M, Giannini R, Marchetti I, Barani L, Basolo F, Ferrannini E, Miccoli P (2009) Thiazolidinediones and antiblastics in primary human anaplastic thyroid cancer cells. Clin Endocrinol (Oxf) 70:946–953
Warburg O (1961) On the facultative anaerobiosis of cancer cells and its use in chemotherapy. Munch Med Wochenschr 103:2504–2506
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Moreno-Sanchez R, Rodriguez-Enriquez S, Saavedra E, Marin-Hernandez A, Gallardo-Perez JC (2009) The bioenergetics of cancer: is glycolysis the main ATP supplier in all tumor cells? Biofactors 35:209–225
Warner TD, Mitchell JA (2004) Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J 18:790–804
Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA (1997) Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 272:3406–3410
Michael MS, Badr MZ, Badawi AF (2003) Inhibition of cyclooxygenase-2 and activation of peroxisome proliferator-activated receptor-gamma synergistically induces apoptosis and inhibits growth of human breast cancer cells. Int J Mol Med 11:733–736
Sun WH, Chen GS, Ou XL, Yang Y, Luo C, Zhang Y, Shao Y, Xu HC, Xiao B, Xue YP, Zhou SM, Zhao QS, Ding GX (2009) Inhibition of COX-2 and activation of peroxisome proliferator-activated receptor gamma synergistically inhibits proliferation and induces apoptosis of human pancreatic carcinoma cells. Cancer Lett 275:247–255
Hau P, Kunz-Schughart L, Bogdahn U, Baumgart U, Hirschmann B, Weimann E, Muhleisen H, Ruemmele P, Steinbrecher A, Reichle A (2007) Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-gamma agonists in recurrent high-grade gliomas—a phase II study. Oncology 73:21–25
Reichle A, Bross K, Vogt T, Bataille F, Wild P, Berand A, Krause SW, Andreesen R (2004) Pioglitazone and rofecoxib combined with angiostatically scheduled trofosfamide in the treatment of far-advanced melanoma and soft tissue sarcoma. Cancer 101:2247–2256
Carlsson J, Yuhas JM (1984) Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res 95:1–23
Sutherland RM (1988) Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 240:177–184
Kunz-Schughart LA, Kreutz M, Knuechel R (1998) Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. Int J Exp Pathol 79:1–23
Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, Oh W, Demetri G, Figg WD, Zhou XP, Eng C, Spiegelman BM, Kantoff PW (2000) Effects of ligand activation of peroxisome proliferator-activated receptor gamma in human prostate cancer. Proc Natl Acad Sci U S A 97:10990–10995
Arah IN, Song K, Seth P, Cowan KH, Sinha BK (1998) Role of wild-type p53 in the enhancement of camptothecin cytotoxicity against human prostate tumor cells. Anticancer Res 18:1845–1849
Brown J (1962) Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism 11:1098–1112
Al Taie OH, Graf T, Illert B, Katzenberger T, Mork H, Kraus MR, Barthelmes HU, Scheurlen M, Seufert J (2009) Differential effects of PPARgamma activation by the oral antidiabetic agent pioglitazone in Barrett’s carcinoma in vitro and in vivo. J Gastroenterol 44:919–929
Strakova N, Ehrmann J, Bartos J, Malikova J, Dolezel J, Kolar Z (2005) Peroxisome proliferator-activated receptors (PPAR) agonists affect cell viability, apoptosis and expression of cell cycle related proteins in cell lines of glial brain tumors. Neoplasma 52:126–136
Rodriguez-Enriquez S, Gallardo-Perez JC, Aviles-Salas A, Marin-Hernandez A, Carreno-Fuentes L, Maldonado-Lagunas V, Moreno-Sanchez R (2008) Energy metabolism transition in multi-cellular human tumor spheroids. J Cell Physiol 216:189–197
Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP (1998) Ligand for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res 58:3344–3352
Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C (2004) Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 53:1052–1059
Coates G, Nissim I, Battarbee H, Welbourne T (2002) Glitazones regulate glutamine metabolism by inducing a cellular acidosis in MDCK cells. Am J Physiol Endocrinol Metab 283:E729–E737
Dello RC, Gavrilyuk V, Weinberg G, Almeida A, Bolanos JP, Palmer J, Pelligrino D, Galea E, Feinstein DL (2003) Peroxisome proliferator-activated receptor gamma thiazolidinedione agonists increase glucose metabolism in astrocytes. J Biol Chem 278:5828–5836
Artwohl M, Furnsinn C, Waldhausl W, Holzenbein T, Rainer G, Freudenthaler A, Roden M, Baumgartner-Parzer SM (2005) Thiazolidinediones inhibit proliferation of microvascular and macrovascular cells by a PPARgamma-independent mechanism. Diabetologia 48:586–594
Singh D, Banerji AK, Dwarakanath BS, Tripathi RP, Gupta JP, Mathew TL, Ravindranath T, Jain V (2005) Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol 181:507–514
Crokart N, Radermacher K, Jordan BF, Baudelet C, Cron GO, Gregoire V, Beghein N, Bouzin C, Feron O, Gallez B (2005) Tumor radiosensitization by antiinflammatory drugs: evidence for a new mechanism involving the oxygen effect. Cancer Res 65:7911–7916
Acknowledgments
This project was supported in part by the Deutsche Forschungsgemeinschaft (Krm-1418/6-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gottfried, E., Rogenhofer, S., Waibel, H. et al. Pioglitazone modulates tumor cell metabolism and proliferation in multicellular tumor spheroids. Cancer Chemother Pharmacol 67, 117–126 (2011). https://doi.org/10.1007/s00280-010-1294-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1294-0