Abstract
Purpose
5-Fluorouracil (5-FU) plus cisplatin (C) can be considered a standard option for advanced gastric cancer (AGC). Irinotecan (Ir) and docetaxel (D) are active agents with no complete cross-resistance with C and 5-FU. Concomitant combination of Ir or D with C and 5-FU is feasible, but with substantial toxicities. A different way to include all active agents in first-line treatment of AGC may be to use them sequentially. We aimed to evaluate the activity and the safety profile of sequential chemotherapy with 5-FU-based doublets with C, Ir and D in the first-line treatment of AGC.
Methods
We conducted a phase II study of first-line sequential chemotherapy in metastatic GC. Treatment consisted of 3 cycles of C + infused 5-FU and leucovorin (CFL) followed by 3 cycles of Ir + 5-FU/LV (IrFL) followed by 3 cycles of D + 5-FU/LV (DFL). Primary end-point was response rate.
Results
Forty-six patients were enrolled, median age 60 years, sites of disease (single/multiple) = 9/37, PS 0/1 = 27/19, gastric/gastro-oesophageal junction = 39/7. Median number of cycles was 9. Main grade 3–4 toxicities were neutropenia (37%), febrile neutropenia (2%), diarrhoea (4%), stomatitis (9%). Response rate after the planned 9 cycles was 45% (15 partial and 5 complete responses among 43 evaluable patients). Median PFS and OS: 6.8 and 11.1 months, respectively.
Conclusion
This sequential treatment is feasible with a favourable safety profile and produced encouraging results in terms of activity and efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth most common cancer worldwide [1]. While fundus and distal gastric cancer incidence declines, the rate of gastro-oesophageal junction adenocarcinoma is rising [2]. Cure for patients with gastric cancer is only available for those in whom a complete surgical resection can be performed. Advanced disease is incurable, and treatment is based on palliative chemotherapy [3]. A meta-analysis of studies comparing polychemotherapy to single-agent treatment showed a significant gain in median survival with combination therapy establishing its role in the palliative setting [4]. A number of different chemotherapy combination regimens has been developed using the most active agents in advanced and metastatic gastric cancer without establishing a universally accepted standard regimen [5]. On the other hand, the combination of 5-fluorouracil (5-FU) and cisplatin remains an accepted treatment option for this disease [6] both in Europe and North America. The addition of an anthracycline is a widely accepted option since a meta-analysis [4] that compared 5-FU/cisplatin-containing regimens with versus without anthracyclines (HR = 0.77, 95% CI 0.62–0.95) showed a significant (but relatively small) survival benefit for the three-drug combination.
The disappointing results in terms of both progression-free survival (PFS) and overall survival (OS), around 5 and 9 months, respectively, achieved by conventional treatment regimens have sustained the investigation of new drugs. Irinotecan and docetaxel are active agents in gastric cancer and have been already tested in randomized phase II and III studies.
A recent phase III randomized trial compared the combination of irinotecan plus 5-FU (IF) to cisplatin plus 5-FU [7], demonstrating that IF was not inferior to the standard doublet in terms of time to progression (TTP) in the full-analysis population and resulted in a different toxicity profiles compared to cisplatin-based treatment, thus representing a possible platinum-free alternative in the first-line treatment of advanced gastric cancer (AGC).
Docetaxel is the last chemotherapic agent that granted approval for first-line treatment of AGC. This was achieved for the results of the TAX-V325 phase III randomized study, that randomly assigned AGC patients to the triplet combination of docetaxel, cisplatin and 5-FU (DCF) or cisplatin plus fluorouracil [8]. The taxane improved response rate, TTP and OS, but with a substantial increase in toxicity [6, 9]. Such considerations limit the routinary adoption of DCF as a standard first-line for advanced or metastatic gastric cancer [10].
Irinotecan and docetaxel have different mechanisms of action and a non-complete cross-resistance with cisplatin and 5-FU, but their concomitant administration is unfeasible.
A different way of including all most active agents in the first-line treatment of AGC with a strong experimental rationale is to use them sequentially to the other active regimens. The use of docetaxel sequential to an intensive weekly PELF regimen has been already studied in gastric cancer with good activity results and an acceptable safety profile [11].
Moving from the above reported considerations, we conducted a phase II study to evaluate the feasibility and the antitumoural activity of a sequential regimen involving three different combinations of cisplatin, irinotecan and docetaxel based on an infused 5-FU plus folinic acid treatment repeated every 2 weeks.
Patients and methods
Patient selection
Eligible patients were required to have histologically confirmed metastatic adenocarcinoma of the stomach or of the gastro-oesophageal junction, at least one measurable lesion according to response evaluation criteria in solid tumors (RECIST) criteria, age between 18 and 75 years, an Eastern Cooperative Oncology Group performance status = 1 or less for patients ≤70 years and = 0 for patients aged 71–75, a life expectancy of at least 3 months, no prior palliative chemotherapy (previous adjuvant and/or neoadjuvant chemotherapy was allowed if more than 12 months have elapsed between the end of adjuvant and/or neoadjuvant therapy and first relapse), at least 6 weeks elapsed from prior radiotherapy and 3 weeks from surgery, adequate bone marrow, hepatic and renal function (haemoglobin ≥9.0 g/dl, absolute neutrophil count ≥2.0 × 109/l, no blood transfusions within 2 weeks, platelet count ≥100 × 109/l, creatinine ≤1.25 the upper limits of normal (ULN), total bilirubin ≤1.5 × ULN, aspartate aminotransferase and alanine aminotransferase ≤1.5 × ULN, alkaline phosphatase ≤2.5 × UNL, except in case of bone metastasis without any liver disease). Patients who had received prior treatment with platinum, camptothecins or taxanes, patients with metastases to the central nervous system, prior history of another malignancy within 5 years of study entry except for basal cell carcinoma of the skin or carcinoma in situ of the uterine cervix, patients with bowel obstruction (or subobstruction) or history of inflammatory enteropathy or extensive intestinal resection (>hemicolectomy or extensive small intestine resection with chronic diarrhoea) were excluded from the study. All participants provided written informed consent before they entered the study, which was approved by local Ethics Committee.
Treatment
The planned treatment consisted of a total of 9 cycles of chemotherapy repeated every 2 weeks. Figure 1 reproduces a diagram of treatment schedule. The first 3 cycles consisted of cisplatin 50 mg/m² intravenous infusion (IV) over 30 min on day 1 immediately followed by leucovorin (LV) 200 mg/m² over 2 h immediately followed by 5-FU 3.200 mg/m² infused as a 48-h continuous infusion (CFL). Cycles fourth to sixth consisted of irinotecan 180 mg/m² IV over 60 min on day 1 immediately followed by LV 200 mg/m² over 2 h immediately followed by 5-FU 3.200 mg/m² infused as a 48-h continuous infusion (IrFL). The last 3 cycles consisted of docetaxel 50 mg/m² IV over 60 min on day 1 immediately followed by LV 200 mg/m² over 2 h immediately followed by 5-FU 3.200 mg/m² infused as a 48-h continuous infusion (DFL).
Activity, efficacy and toxicity assessment
Pre-treatment evaluation included history and physical examination, PS assessment, complete blood cell with differential and platelet counts, complete blood profile, carcinoembryonic antigen, electrocardiogram, computed tomography (CT) scan of abdomen and chest. During treatment, a physical examination and a complete blood cell count were performed every 2 weeks. Toxic effects were monitored weekly and were scored according to standard NCI CTC criteria.
Every 3 cycles, sites of metastases were re-evaluated by means of CT scan (i.e. at the end of each sequential doublet) and a complete blood profile was performed. Responses were evaluated according to RECIST [12]. A CT scan was performed every 8 weeks after the end of treatment until evidence of progressive disease. Patients who received at least 1 cycle of treatment were considered assessable for response and toxicity. PFS was calculated from the first day of treatment to the date on which disease progression was first documented or to the date of death or of the last follow-up. OS was calculated from the first day of treatment to the date of death or last follow-up.
Statistical considerations
The primary endpoint was the cumulative objective response rate (complete plus partial responses according to RECIST) after the planned 9 cycles of chemotherapy. Secondary endpoints were response rates after 3 cycles of every single doublet (cisplatin/5-FU, irinotecan/5-FU, docetaxel/5-FU), progression-free survival, overall survival and toxicity profile.
The optimum two-stage sequential design described by Simon [13] was used to determine the number of patients to be included. Because responses with most widely used standard combinations of 5-FU and cisplatin are observed in ~30% of patients, a response rate of ~50% for a new regimen that has acceptable toxic effects would be considered promising. Therefore, the design parameters p0 (response rate in null hypothesis) and p1 (response rate in alternative hypothesis) selected were 0.30 and 0.50, respectively. Considering in addition an alpha and beta error probability of 0.05 and 0.20, the first stage of the study required 15 patients, and if at least 5 objective responses were observed, the second stage required a total of 46 patients. If at least 18 patients responded after the second accrual stage, treatment was considered promising unless other considerations indicated otherwise. The distribution of PFS and OS were calculated using the Kaplan–Meier method.
Results
Patient characteristics
A total of 46 patients were enroled. The baseline characteristics are listed in Table 1. The median age was 61 years (range 37–75 years). Thirty-nine patients (85%) had a gastric adenocarcinoma and 7 patients (15%) had a gastro-oesophageal junction adenocarcinoma. All patients had distant metastases and no patients had a locally advanced disease. Thirty-seven patients (80%) had two or more sites of metastases. Twenty-six (57%) had liver involvement, 5 (11%) had lung metastases, 23 (50%) had distant lymph node metastases and 17 (37%) peritoneal dissemination.
Response
In total, 330 cycles were administered with a median of 9 cycles per patient. Forty-three (93.5%) of 46 patients were assessable for response. Three patients were not assessable for response because they did not undergo regular CT scans. The overall response rate at the end of the sequential treatment (ORR) was 45% (95% CI, 30 to 60%) by per-protocol analysis. There were 5 complete responses (CRs) and 15 partial responses (PRs). With regard to responses to each single doublet (i.e. responses assessed comparing CT scans performed after each doublet with those performed before starting that doublet) were as follows: 10 out of 40 evaluable patients responded to CFL (25%; 95% CI, 12 to 38%, 6 patients were not assessable for response to CFL), 24 obtained a disease stabilization while 6 progressed. Thirteen out of 35 patients responded to IrFL (37%; 95% CI, 21 to 53%, 7 patients did not receive IrFL and 4 were not assessable for response to IrFL), 18 obtained a disease stabilization while 4 progressed. Seven out of 34 patients responded to DFL (21%; 95% CI, 7 to 34%, 9 patients did not receive DFL and 3 were not assessable for response to DFL), 21 obtained a disease stabilization while 6 progressed. Global activity results and responses to each doublet are resumed in Table 2. Upon disease progression, 23 patients (50%) received a second-line treatment, including an anthracyclin (n = 10), oxaliplatin (n = 5) or one of the doublets already received in first-line (n = 8), judged by the investigator the most active and/or better tolerated. Among these last 8 patients, 6 received again the complete sequence of 9 cycles as in first-line.
Progression-free and overall survival
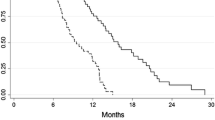
All patients were included in the survival analysis. The median follow-up duration was 23 months. The median PFS was 6.8 months and the median survival was 11.1 months (Fig. 2).
Toxicity
All patients were assessable for safety. Table 3 summarizes main toxicities per patient. There were no unexpected toxicities. Main grade 3–4 toxic effects were as follows: 2% of patients experienced grade 3 nausea, 3% grade 3 and 1% grade 4 diarrhoea, 7% grade 3 and 2% grade 4 stomatitis, 27% grade 3 and 11% grade 4 neutropenia.
There was one treatment-related death after the first cycle of CF in a 69-year-old man due to pneumonitis and septicaemia developed after grade 4 stomatitis, grade 3 diarrhoea and grade 4 neutropenia. No other patients were discontinued from the study due to toxic effects. Five (11%) patients required dose reduction of at least one drug and 7 (15%) were delayed >1 week due to grade 3 or 4 side effects. Prophylactic G-CSF was adopted for subsequent cycles due to grade 4 neutropenia lasting >7 days in one patient after the first administration of DFL.
Discussion
Prognosis of AGC is dismal, and the main role of chemotherapy is to offer the best palliation. This means a balance between survival prolongation and symptoms’ relief and improvement in quality of life, so that toxicity profile of adopted treatments is of major importance. The combination of 5-FU and cisplatin still represents an accepted compromise between efficacy and tolerability, so that it has been chosen as standard comparator arm in many ongoing or recently closed phase III trials [14–16].
Three randomized trials tested treatment combinations of infused 5-FU and irinotecan [7, 17, 18]. A phase III trial randomized 333 patients to irinotecan plus high-dose 5-FU and leucovorin (IF) or cisplatin and 5-FU (CF). The IF regimen was not superior over CF for time to progression (5.0 versus 4.2 months), median OS time 9.0 versus 8.7 months, or RR 31.8% versus 25.8%, but showed a better safety profile: in fact, while increased rate of grade 3–4 diarrhoea was reported with IF, the incidence of grade 3–4 stomatitis and haematological toxicity, in particular neutropenic fever, was significantly lower. [7]. According to a meta-analysis, irinotecan regimens show a non-statistically significant benefit in survival of about 1 month and a lower rate of treatment-related deaths over the reference regimen [3]. Taken together, all these data suggest that irinotecan is efficacious almost as cisplatin, but has a different safety profile.
The TAX-V325 trial [8], which established the role of docetaxel combinations in AGC, demonstrated a significant increase in response rate (37 vs. 25%), in time to progression (5.6 vs. 3.7 months) and in overall survival (9.2 vs. 8.6, 23% risk reduction and 18 vs. 9% of patients alive at 2 years) for DCF over CF. The cost for such advantages was high in terms of toxicity: in DCF arm, the rate of G3-4 diarrhoea, G3-4 neutropenia and febrile neutropenia was 19, 82 and 29%, respectively, and significantly higher than with CF (8, 57 and 12%, respectively). Moreover, 21% of patients treated with DCF experienced G3-4 stomatitis and from these data, it is derived that in many patients such adverse events were concomitant and possibly life-threatening. Even if toxic deaths were not increased by DCF, the rate of hospitalization was not reported. Another reason for concern is that these results have been obtained in a rather selected population with good performance status (only 1% of the enroled patients had a Karnofsky performance status of 70) and low median age (55 years).
Given its spare feasibility, many experts questioned the role of DCF as optimal first-line therapy [9, 10]. Meanwhile, in routinary practice, oncologists claim for a more manageable scheme that could be more quietly administered to unselected patients without the need for dose adjustments or delays that inevitably raise concerns about the efficacy of treatment in each single patient. For such reasons, many investigators studied modifications of the DCF regimen [19, 20]: weekly schedules or split-doses of docetaxel lead to results that compare favourably with those of DCF in terms of activity and efficacy, but with an improved safety profile, especially for a low incidence of grade 3–4 and febrile neutropenia (less than 5%).
In our study, we evaluated a sequential strategy incorporating irinotecan and docetaxel in addition to 5-FU and cisplatin in the first-line therapy of metastatic gastric cancer with RR as main objective. Primary end-point was met according to the statistical hypothesis. The median progression-free survival of 6.8 months and the median OS of 11.1 months compare favourably with those obtained with other regimens in major phase III trials, in particular, considering that our population was clearly unselected and representative of metastatic gastric patients who come to our attention in routinary clinical practice. It has been suggested that low median age and a good performance status may have positively affected the results of TAX-V325 study [9, 10], and this was not the case of our series. At the same time, there were no patients with locally advanced disease in our experience, while they represented a significant subgroup (~22%) in the REAL-2 study [21] that for the first time reported a median OS of more than 11 months for the epirubicin, capecitabine and oxaliplatin arm, and this may have positively affected such results. The safety profile of the sequential treatment was extremely favourable with no toxicities exceeding the incidence of 9% except for neutropenia that was febrile only in one case. That patient died as consequence of treatment-related toxicity, but this occurred after the first cycle of 5-FU/cisplatin and therefore cannot be ascribed to the sequential strategy, nevertheless this is consistent even with most recent series of patients treated with such combination, reporting 1–5% incidence of toxic deaths, mainly for infections [8, 14].
One can argue that a possible limitation of our study could reside in the choice of switching to a different regimen in patients responding to the previous doublet after only three cycles. However, in our opinion, this does not preclude the use of the most active and better tolerated doublet at the end of the planned 9 cycles. Activity and feasibility of this strategy seems to be confirmed by results obtained in the 10 patients of our study exposed in second-line to a previously received doublet, deemed the best at investigator’s choice. Meanwhile, it should be noted that activity of treatment seemed to be progressively improved throughout the course of sequential treatment, in fact only 2 previously responding patients progressed at the switch of the doublet, while 14 improved their response and 4 of them gradually obtained the complete disappearance of all target lesions. In conclusion, in the present phase II trial, a sequential first-line treatment with infused 5-FU-based doublets containing cisplatin, irinotecan and docetaxel led to interesting results in terms of activity and efficacy with a very favourable toxicity profile in comparison with standard first-line regimens, especially if considering those containing docetaxel. In particular, our regimen, other than being a possible choice particularly for patients at higher risk of toxicity with three-drugs combination regimens, could be considered as a possible chemotherapic platform for further phase II randomized studies with the aim to investigate the addition of new molecularly targeted agents.
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Van Cutsem E, Van de Velde C, Roth A et al (2008) Expert opinion on management of gastric and gastro-oesophageal junction adenocarcinoma on behalf of the European organisation for research and treatment of cancer (EORTC)-gastrointestinal cancer group. Eur J Cancer 44:182–194
Catalano V, Labianca R, Beretta GD et al. (2009) Gastric cancer. Crit Rev Oncol Hematol 71:127–164
Wagner AD, Grothe W, Haerting J et al (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24:2903–2909
Ajani JA (2006) Standard of care for gastric cancer based on meta-analysis? Treading on thin ice or it is very nice!. J Clin Oncol 24:5473–5474 author reply 5474-5476
Wong R, Cunningham D (2009) Optimising treatment regimens for the management of advanced gastric cancer. Ann Oncol 20:605–608
Dank M, Zaluski J, Barone C et al (2008) Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 19:1450–1457
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 study group. J Clin Oncol 24:4991–4997
Wagner AD, Wedding U, Kuss O, Hoffken K (2007) Docetaxel for advanced gastric cancer? J Clin Oncol 25:2490–2491 author reply 2491-2493
Ilson DH (2007) Docetaxel, cisplatin, and fluorouracil in gastric cancer: does the punishment fit the crime? J Clin Oncol 25:3188–3190
Cascinu S, Graziano F, Barni S et al (2001) A phase II study of sequential chemotherapy with docetaxel after the weekly PELF regimen in advanced gastric cancer. A report from the Italian group for the study of digestive tract cancer. Br J Cancer 84:470–474
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Kang YK, Kang WK, Shin DB et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20:666–673
A study of bevacizumab in combination with capecitabine and cisplatin as first-line therapy in patients with advanced gastric cancer. Source: www.cancer.gov. Accessed 15 Sep 2009
Erbitux in combination with xeloda and cisplatin in advanced esophago-gastric cancer. Soruce: www.cancer.gov. Accessed 15 Sep 2009
Bouche O, Raoul JL, Bonnetain F et al (2004) Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study–FFCD 9803. J Clin Oncol 22:4319–4328
Moehler M, Eimermacher A, Siebler J et al (2005) Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer 92:2122–2128
Lorenzen S, Hentrich M, Haberl C et al (2007) Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol 18:1673–1679
Tebbutt N, Sourjina T, Strickland A et al. (2007) ATTAX: Randomised phase II study evaluating weekly docetaxel-based chemotherapy combinations in advanced esophago- gastric cancer, final results of an AGITG trial. J clin Oncol, ASCO Annual Meeting Proceedings 25: 4528
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Acknowledgments
This study was presented in part at the 31st European Society for Medical Oncology Congress, Istanbul, Turkey, 29 September to 3 October 2006. We would like to thank Michele Andreuccetti, Dr. Eva Betjia and Dr. Simona Giannace for data collection and administrative support. We acknowledge Rhone-Poulenc Rorer, Inc. who supplied Irinotecan (Campto®) and Docetaxel (Taxotere®) for the study.Granting Sponsor: Gruppo Oncologico Nord-Ovest (G.O·N.O.).
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Loupakis and G. Masi contributed equally to the study.
Rights and permissions
About this article
Cite this article
Loupakis, F., Masi, G., Fornaro, L. et al. Phase II study of sequential cisplatin plus 5-fluorouracil/leucovorin (5-FU/LV) followed by irinotecan plus 5-FU/LV followed by docetaxel plus 5-FU/LV in patients with metastatic gastric or gastro-oesophageal junction adenocarcinoma. Cancer Chemother Pharmacol 66, 559–566 (2010). https://doi.org/10.1007/s00280-009-1196-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1196-1