Abstract
Purpose
We performed a retrospective study to evaluate the efficacy of cetuximab plus chemotherapy in metastatic gastric cancer (MGC) patients previously treated with chemotherapy and to investigate potential predictors of treatment efficacy in those patients.
Methods
Thirty-two patients with MGC were included in this study. Cetuximab was delivered, often combined with irinotecan-based chemotherapy. Thirty patients were analyzed for K-ras mutations via direct sequencing of the tumor DNA.
Results
Patients were heavily pretreated with a median number of three previous lines of palliative chemotherapy (56% of the patients were refractory to all of the following drugs: fluoropyrimidines, cisplatin, irinotecan, oxaliplatin, and docetaxel) and 53% of the patients displayed poor performance status. Of 28 response-assessable patients, the overall response rate to cetuximab plus chemotherapy was 3.6% [95% confidence interval (CI) 0–10.5%] and the disease control rate was 28.6%. The median progression-free survival (PFS) was 1.7 months (95% CI 1.3–2.1 months), and the median overall survival (OS) was 3.2 months (95% CI 1.4–5.0 months). Multivariate analyses revealed that skin rash and performance status were significantly associated with PFS and OS. The presence of a K-ras mutation (13.3%) was not associated with either PFS or OS.
Conclusion
Our study suggests that MGC patients with good performance status and skin rash benefit most from salvage cetuximab combined with chemotherapy, even in heavily pretreated status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite its decreasing incidence over the past few decades, gastric cancer remains a global health issue; it is the second most common cause of cancer deaths worldwide [1]. Although a survival benefit has been demonstrated by systemic chemotherapy in patients with locally advanced or metastatic gastric cancer (MGC) [2, 3], the benefits of combination chemotherapy have been modest, even with modern agents such as oral fluoropyrimidines, irinotecan, oxaliplatin, and taxanes [4–9]. Therefore, more effective treatments are needed to improve survival in these patients.
Recently, an increased understanding of the molecular basis of cancer has led to the development of specific molecule-targeted agents. One example is the epidermal growth factor receptor (EGFR), a member of the tyrosine kinase growth factor receptor superfamily that is overexpressed in a variety of cancers, including gastric cancer (10.4–63%) [10–14]. In addition, EGFR overexpression is associated with tumor progression and poor prognosis in gastric cancer [11, 12, 14]. EGFR expression was, however, a positive prognostic factor for overall survival (OS) in gastric cancer patients who underwent curative resection followed by adjuvant chemotherapy with 5-fluorouracil and cisplatin; relative risk of death 0.475 [95% confidence interval (CI) 0.282–0.791], P = 0.005 [15]. The variations of the results between different studies might be due to heterogeneity of EGFR expression within the tumor and different methodological setups. Several techniques can be used for measuring EGFR expression, including immunohistochemistry (IHC), reverse transcriptase polymerase chain reaction (PCR), and fluorescence in situ hybridization (FISH). Although IHC is the most widely used method for determining EGFR expression, its value is limited by lack of methodological standardization. In addition, there has been a controversial relationship between EGFR protein expression as detected by IHC and gene copy number detected by FISH [15, 16]. The prognostic role of EGFR expression and the best approach to measuring EGFR in gastric cancer need to be further validated.
Agents targeting EGFR, such as cetuximab, are in clinical use. Cetuximab, a chimeric IgG1 monoclonal antibody (mAb) directed at the EGFR binding site, has demonstrated activity in a variety of cancers, including colorectal cancer and head and neck cancer, in both first-line and refractory settings [17, 18]. Interestingly, skin toxicity that evolves during the first weeks of cetuximab treatment has been identified as a potent predictor of the response to it [19, 20]. The K-ras mutation has recently been demonstrated as a predictive factor for resistance to cetuximab [21–24].
In gastric cancer, several phase II trials of cetuximab plus chemotherapy have shown promising results, mainly in chemo-naive patients with advanced gastric or gastroesophageal junction adenocarcinoma [25–32]. However, limited data on the efficacy and predictors for success of cetuximab salvage therapy are available for heavily pretreated patients with gastric cancer.
The aim of this study was (1) to evaluate the efficacy of cetuximab-containing therapy in MGC patients previously treated with one or more chemotherapy regimens and (2) to investigate predictors of treatment efficacy in those patients.
Patients and methods
Patients and treatment
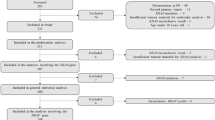
We retrospectively reviewed 32 consecutive MGC patients who had received cetuximab plus chemotherapy as salvage therapy after failure of one or more systemic cytotoxic chemotherapies between July 2006 and June 2008 at the Research Institute and Hospital, National Cancer Center, Korea. All of these patients had a histologically proven gastric adenocarcinoma.
Cetuximab was delivered at an initial dose of 400 mg/m2 i.v., followed by weekly doses of 250 mg/m2, most often along with a combination of irinotecan-based chemotherapy: weekly irinotecan (n = 24), biweekly irinotecan/infusional 5-fluorouracil (5-FU)/leucovorin (LV) (n = 2), biweekly irinotecan/cisplatin (n = 1), biweekly oxaliplatin/5-FU/LV (n = 3), or docetaxel ± cisplatin (n = 2). Treatment was continued until disease progression, unacceptable toxicity, or patient’s refusal.
Chemotherapy was delayed until absolute neutrophil counts were ≥1.5 × 109 L−1 (or 1.0 × 109 L−1 if leukocyte counts were ≥3.0 × 109 L−1), platelet counts were ≥75 × 109 L−1, and other non-hematological toxicity was ≤grade 1. Chemotherapy doses were reduced by 20% for grade 4 myelosuppression, grade 3/4 febrile neutropenia or non-hematological toxicity, or recurrent grade 2 diarrhea or abdominal pain. Administration of granulocyte-colony stimulating factor was permitted in patients with grade 4 neutropenia or grade 3/4 febrile neutropenia.
An institutional review board at the Research Institute and Hospital, National Cancer Center, Korea, reviewed and approved this study. Patient consent for analysis of stored biological samples and relevant clinical data were verified for all patients included.
Evaluation of treatment efficacy
Tumor response was evaluated by computed tomography (CT) scan according to the Response Evaluation Criteria in Solid Tumors (RECIST) [33]. CT scans were performed within 4 weeks of treatment initiation and were repeated every 6–8 weeks during treatment. Responses were confirmed by subsequent CT scans at least 4 weeks after documentation of the initial response. Skin toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0. These criteria indicated that an acne-like skin rash not requiring intervention was grade 1, that requiring intervention was grade 2, and that being associated with pain, disfigurement, ulceration, or desquamation was grade 3.
DNA extraction and K-ras mutation analyses
K-ras mutation analyses were performed in 30 patients and the remaining 2 patients were excluded because tumor samples were unavailable. Tumor samples were obtained from the primary gastric tumor (n = 25), metastatic tissue (n = 2), or both (n = 3).
DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissue sections with the QIAmp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. For FFPE tissues, samples were obtained after histologic verification of the presence of tumor cells (>70%) in each tumor sample.
The presence of K-ras point mutations was assessed in codons 12 and 13, two hotspots that include more than 95% of the mutations in this gene [34]. The primers used for K-ras gene analysis were 5′-ACTGAATATAAACTTGTGGTAGTTGGACCT-3′ (forward) and 5′-TCAAAGAATGGTCCTGGACC-3′ (reverse). The PCR reaction mixture (20 μl) contained approximately 0.1 μg of genomic DNA in 20 mM Tris hydrochloride (pH 8.4), 50 mM potassium chloride, 2.0 mM magnesium chloride, 0.2 mM each of deoxyribonucleoside triphosphate, 0.2 μM of each primer, and 5 U of f-Taq DNA polymerase (Solgent, Daejeon, Korea). Templates were initially denatured for 5 min at 94°C, followed by 35 cycles of PCR with incubations of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C. The reaction was incubated at 72°C for 5 min during the last cycle. PCR reactions were checked for appropriate amplification by running the DNA fragments in an ethidium bromide-stained agarose gel and visualizing the bands by UV light transillumination.
PCR products were purified with a QIAquick PCR purification kit (Qiagen, Valencia, USA) and then sequenced with an ABI3130x1 DNA sequencer (Applied Biosystems, Foster City, USA) using dye-primer conditions recommended by the manufacturer. In each case, the detected mutation was confirmed in the sequence of both the sense and antisense strands.
Statistical methods
Because the present study was a retrospective analysis, formal estimation of the sample size and calculation of the power were not performed. The Fisher’s exact test was used to calculate the P value for the association between patient characteristics, K-ras mutations, skin toxicity, and disease control rate of treatment.
Progression-free survival (PFS) was calculated from the start of the study treatment to the date of progression or last follow-up. OS was calculated from the first day of the study treatment to the day of death or last follow-up. Both PFS and OS were estimated using the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis using the Cox proportional hazards model was used to estimate the simultaneous effects of prognostic factors for survival. The differences were considered to be statistically significant when P ≤ 0.05.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. There were 18 (56%) males and 14 (44%) females, and the median age of the patients was 52 years (range 28–82 years). Seventeen (53%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or 3, and most patients (81%) had multiple metastases involving two or more organ systems. Patients were heavily pretreated with a median number of three previous lines of palliative chemotherapy (range 1–6) (Table 1). Eighteen patients (56%) had been previously exposed to all of the following classes of drugs: fluoropyrimidines, cisplatin, irinotecan, oxaliplatin, and docetaxel. All patients had been refractory to prior chemotherapy, which was defined as progression while receiving chemotherapy.
K-ras mutation
Sequencing of K-ras codons 12 and 13 was performed in 30 patients, among whom 4 patients (13.3%) displayed a K-ras mutation: G12D (n = 1) and G12V (n = 1) at codon 12, G13C (n = 1) and G13V (n = 1) at codon 13. Among three patients in whom tumor samples were available from both primary gastric tumor site and metastatic site, two patients had the same K-ras mutation status between two sites and one patient showed the discordant result.
Treatment administration
Median number of chemotherapy cycle was 2 (range 1–7) and the median week of cetuximab treatment was 6 weeks (range 1–28). Median dose intensity of cetuximab, irinotecan, oxaliplatin, and docetaxel was 1.00 (range 0.97–1.00), 0.91 (range 0.50–1.00), 0.98 (range 0.94–1.00), and 0.97 (range 0.95–1.00), respectively.
Response
Of the 32 patients, 28 were assessed for tumor response. Four patients could not be assessed for overall response rate because one patient died as a result of asphyxia during week 2 of treatment and three patients refused treatment after just 1 week (n = 1) or 2 weeks of treatment (n = 2). One patient (3.6%) achieved a partial response, 7 patients (25.0%) displayed stable disease, and 20 patients (71.5%) experienced disease progression. The overall response rate was 3.6% (95% CI 0–10.5%), and the disease control rate (partial response plus stable disease) was 28.6%. When the differences in disease control rate were evaluated by patient characteristics, prior therapy, and the type of chemotherapy administered concomitantly with cetuximab, there was a significant difference in the disease control rate based on ECOG performance status (1 vs. ≥2, 53.8 vs. 6.7%; P = 0.01) (Table 2). An acne-like skin rash was observed in 50% of the patients, among whom 11 (34%) presented a grade 1 rash and 5 (16%) presented a grade 2 skin rash. Skin rash was also significantly associated with the disease control rate (grade 0–1 vs. 2, 17.4 vs. 80.0%; P = 0.01). K-ras mutation, however, was not associated with the disease control rate (wild-type vs. mutant, 36.8 vs. 25.0%; P = 1.00).
Survival
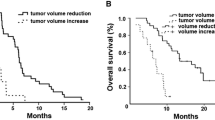
All 32 patients were evaluated for survival analysis. The median duration of follow-up was 18.8 months (range 7.0–30.4 months). The median PFS was 1.7 months (95% CI 1.3–2.1 months), and the median OS was 3.2 months (95% CI 1.4–5.0 months) (Fig. 1).
Univariate Cox regression analysis revealed that the ECOG performance status and skin rash were statistically significant factors influencing PFS (Table 3). The median PFS was 1.4 months in patients with poor performance status (ECOG performance status ≥ 2), as compared with 2.4 months in those with good performance status (log-rank P = 0.002) (Fig. 2a). The median PFS of patients with skin rash grade 0 versus grade 1 versus grade 2 was 1.0 versus 1.7 versus 4.0 months, respectively (log-rank P < 0.001) (Fig. 2b).
Multivariate analysis showed that lack of skin rash [hazard ratio (HR) = 5.03; 95% CI 1.29–19.70; P = 0.02] was a significant independent prognostic factor for PFS, and poor ECOG performance status demonstrated only borderline significance (HR = 3.36; 95% CI 0.96–11.79; P = 0.05) (Table 3).
With regard to OS, univariate and multivariate analyses revealed that ECOG performance status (HR = 5.49; 95% CI 2.08–14.50; P = 0.001), the number of metastatic organ sites (HR = 5.68; 95% CI 2.23–14.48; P < 0.001), and skin rash (HR = 6.00; 95% CI 2.34–15.38; P < 0.001) were significant independent prognostic factors (Table 4; Fig. 2c, d).
In a combined analysis of ECOG performance status and skin rash, patients with two favorable prognostic factors (the presence of skin rash and good performance status) had a median PFS of 3.6 months (95% CI 1.6–5.6 months) and OS of 5.7 months (95% CI 4.0–7.3 months), compared with 1.1 months (95% CI 0.5–1.7 months) (HR = 9.9; 95% CI 2.61–37.91; P = 0.001) and 1.6 months (95% CI 1.0–2.2 months) (HR = 3.62; 95% CI 1.49–8.75; P = 0.004) for patients with only one favorable prognostic factor (the presence of skin rash or good performance status) or no favorable prognostic factors (Fig. 3). The presence of a K-ras mutation was not associated with either PFS or OS.
Discussion
Here, we have shown that cetuximab plus mainly irinotecan-based chemotherapy resulted in a 3.6% response rate, 28.6% disease control rate, 1.7 months of median PFS, and 3.2 months of median OS in heavily pretreated MGC patients. Given that most of our patient cohort (91%) had failed two or more prior regimens, that more than 50% of the patients had been refractory to nearly all effective agents including fluoropyrimidines, cisplatin, irinotecan, oxaliplatin, and docetaxel, and that more than 50% of the patients had poor performance status, these results suggest the possible role of cetuximab combined with other chemotherapies as a salvage therapy in these patients. Recently, several phase II trials showed that cetuximab plus chemotherapy, including oxaliplatin/5-FU/LV, irinotecan/5-FU/LV, capecitainbe/cisplatin or oxaliplatin, 5-FU/LV/cisplatin, or oxaliplatin/irinotecan, resulted in a 48.1–68.6% response rate, 5.23–8 months of median time to progression, and 9.5–16 months of median OS in locally advanced or MGC patients as a first-line treatment [25–32]. As a second-line therapy, cetuximab plus docetaxel resulted in a 6% response rate, 2.1 months of median PFS, and 5.3 months of median OS [35].
Because of the high cost and inconvenience of weekly doctor visits for treatment, it is necessary to better define the subpopulation of patients who have the highest chance of benefiting from cetuximab. This goal would likely be more important in the salvage setting when considering possible treatment-related toxicities in heavily pretreated patients. Although the present study comprised a small sample size, our analysis showed that ECOG performance status and skin rash were associated with the disease control rate, PFS, and OS in MGC patients treated with cetuximab combined with chemotherapy in a salvage setting. Patients with good performance status and a skin rash demonstrated better PFS (median PFS 3.6 vs. 1.1 months; P < 0.001) and OS (median OS 5.7 vs. 1.6 months; P = 0.002) than those with poor performance status and/or lack of skin rash. Skin rash appears to be a surrogate marker for cetuximab efficacy regardless of the type of cancer or nature of administration (first-line or salvage) [19, 20, 32, 36, 37]. Although the mechanism underlying the correlation between skin toxicity and treatment efficacy of cetuximab is currently unclear, it has been hypothesized that the rash may reflect the extent of EGFR blockade in both patients and tumors and that it may reflect the patients’ ability to develop an inflammatory reaction in response to cellular damage caused by EGFR inhibition in the skin. Consequently, patients in whom EGFR blockade is able to trigger an inflammatory response might be more likely to develop rash irrespective of the degree of EGFR inhibition in the tumor [38].
Mutation of K-ras which is a predictive factor for cetuximab efficacy in colorectal cancer was found in 13.3% of the analyzed tumors, consistent with previously reported results in gastric cancer, which ranged from 2.8 to 21% [39–43]. These frequencies of K-ras mutations in gastric cancer are lower than those in colorectal cancer, which are about 40% [44]. In contrast to previous results in colorectal cancer [21, 22, 24], K-ras mutation status was not associated with cetuximab treatment efficacy in the present study. This is consistent with the report by Stella et al. [45] stating that K-ras mutation was not associated with the tumor response to cetuximab plus chemotherapy as a first-line treatment in 43 patients with locally advanced or MGC. It is possible that, in gastric cancer, the molecular mechanisms of resistance to cetuximab can be attributed to other process such as the activation of alternative tyrosine kinase receptors that bypass the EGFR pathway (e.g., IGF-1R and c-Met) rather than RAS constitutive activation by K-ras mutation. Recently, serum EGFR ligands were reported to be implicated in responsiveness to cetuximab therapy in gastric cancer [32]. However, the absence of an association between K-ras mutations and treatment efficacy with cetuximab may be a result of the small sample size. In addition, the low incidence of K-ras mutation in gastric cancer may also contribute to the lack of a significant association with therapeutic success. Thus, this result does not absolutely exclude K-ras mutations as a predictive factor for cetuximab efficacy in gastric cancer, but suggests that, in the clinical setting, the role of K-ras mutation status as a marker for selection of patients who are likely to benefit from cetuximab is less useful than in other types of cancer that harbor a higher K-ras mutation frequency, such as colorectal cancer.
To our knowledge, this is the first study to examine the treatment efficacy of cetuximab combined with chemotherapy in the salvage setting for MGC patients and to correlate treatment success with other factors such as skin rash, ECOG performance status, and K-ras mutation status.
In conclusion, our study suggests that MGC patients with good performance status and skin rash may benefit the most from cetuximab combined with other chemotherapy despite being refractory to nearly all effective chemotherapy. A decision whether starting and/or continuing cetuximab with chemotherapy in these patients could be based on performance status as a prognostic factor in advanced gastric cancer and skin rash during early treatment period as a predictive factor. Large-scale prospective studies are needed to confirm these findings.
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Glimelius B, Ekstrom K, Hoffman K et al (1997) Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 8:163–168
Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M (1995) Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 71:587–591
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24:2903–2909
Dank M, Zaluski J, Barone C et al (2008) Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 19:1450–1457
Al-Batran SE, Hartmann JT, Probst S et al (2008) Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 26:1435–1442
Kang Y, Kang WK, Shin DB et al (2006) Randomized phase III trial of capecitabine/cisplatin (XP) vs. continuous infusion of 5-FU/cisplatin (FP) as first-line therapy in patients (pts) with advanced gastric cancer (AGC): efficacy and safety results. Proc Am Soc Clin Oncol 24:183s (abstr LBA4018)
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Takehana T, Kunitomo K, Suzuki S et al (2003) Expression of epidermal growth factor receptor in gastric carcinomas. Clin Gastroenterol Hepatol 1:438–445
Tokunaga A, Onda M, Okuda T et al (1995) Clinical significance of epidermal growth factor (EGF), EGF receptor, and c-erbB-2 in human gastric cancer. Cancer 75:1418–1425
Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH (2008) EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology 52:738–746
Matsubara J, Yamada Y, Hirashima Y et al (2008) Impact of insulin-like growth factor type 1 receptor, epidermal growth factor receptor, and HER2 expressions on outcomes of patients with gastric cancer. Clin Cancer Res 14:3022–3029
Gamboa-Dominguez A, Dominguez-Fonseca C, Quintanilla-Martinez L et al (2004) Epidermal growth factor receptor expression correlates with poor survival in gastric adenocarcinoma from Mexican patients: a multivariate analysis using a standardized immunohistochemical detection system. Mod Pathol 17:579–587
Kim JS, Kim MA, Kim TM et al (2009) Biomarker analysis in stage III-IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: epidermal growth factor receptor (EGFR) associated with favourable survival. Br J Cancer 100:732–738
Hirsch FR, Varella-Garcia M, Bunn PA Jr et al (2003) Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 21:3798–3807
Mahtani RL, Macdonald JS (2008) Synergy between cetuximab and chemotherapy in tumors of the gastrointestinal tract. Oncologist 13:39–50
Vincenzi B, Schiavon G, Silletta M, Santini D, Tonini G (2008) The biological properties of cetuximab. Crit Rev Oncol Hematol 68:93–106
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208
Lievre A, Bachet JB, Boige V et al (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26:374–379
Lievre A, Bachet JB, Le Corre D et al (2006) KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992–3995
Van Cutsem E, Kohne CH, Hitre E et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Bokemeyer C, Bondarenko I, Makhson A et al (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27:663–671
Pinto C, Di Fabio F, Siena S et al (2007) Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study). Ann Oncol 18:510–517
Lordick F, Lorenzen S, Hegewisch-Becker S et al (2007) Cetuximab plus weekly oxaliplatin/5FU/FA (FUFOX) in 1st line metastatic gastric cancer, Final results from a multicenter phase II study of the AIO upper GI study group. Proc Am Soc Clin Oncol 25:204s (abstr 4526)
Zhang X, Xu J, Shen L et al (2009) A phase II study of cetuximab with cisplatin and capecitabine as first-line treatment in advanced gastric cancer. In: Proceedings of American society of clinical oncology 2009 gastrointestinal cancers symposium, 15–17 Jan 2009 (abstr LBA39)
Kim C, Lee J, Ryu M et al (2009) A prospective phase II study of cetuximab in combination with capecitabine and oxaliplatin (XELOX) in patients with metastatic and/or recurrent advanced gastric cancer. In: Proceedings of American society of clinical oncology 2009 gastrointestinal cancers symposium, 15–17 Jan 2009 (abstr 89)
Yeh K, Hsu C, Hsu C et al (2009) Phase II study of cetuximab plus weekly cisplatin and 24-hour infusion of high-dose 5-fluorouracil and leucovorin for the first-line treatment of advanced gastric cancer. In: Proceedings of American society of clinical oncology 2009 gastrointestinal cancers symposium, 15–17 Jan 2009 (abstr 60)
Woell E, Greil R, Eisterer W et al (2008) Oxaliplatin, irinotecan and cetuximab in advanced gastric cancer: first results of a multicenter phase II trial (AGMT Gastric-2). Proc Am Soc Clin Oncol 26:662s (abstr 15587)
AIO Gastric group, Germany, Moehler MH, Trarbach T et al (2008) Cetuximab with irinotecan/Na-Fa/5-FU as first-line treatment in advanced gastric cancer: preliminary results of a nonrandomised multi-centre AIO phase II study. In: Proceedings of American society of clinical oncology 2008 gastrointestinal cancers symposium, 25–27 Jan 2008 (abstract 102)
Han SW, Oh DY, Im SA et al (2009) Phase II study and biomarker analysis of cetuximab combined with modified FOLFOX6 in advanced gastric cancer. Br J Cancer 100:298–304
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors, European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Frattini M, Balestra D, Suardi S et al (2004) Different genetic features associated with colon and rectal carcinogenesis. Clin Cancer Res 10:4015–4021
Tebbutt NC, Sourjina T, Strickland AH et al (2008) ATTAX2: docetaxel plus cetuximab as second-line treatment for docetaxel-refractory oesophago-gastric cancer—final results of a multicentre phase II trial by the AGITG. Proc Am Soc Clin Oncol 26:659s (abstr 15554)
Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA (2005) Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 23:8646–8654
Xiong HQ, Rosenberg A, LoBuglio A et al (2004) Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol 22:2610–2616
Perez-Soler R, Saltz L (2005) Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol 23:5235–5246
Lee SH, Lee JW, Soung YH et al (2003) BRAF and KRAS mutations in stomach cancer. Oncogene 22:6942–6945
Kim IJ, Park JH, Kang HC et al (2003) Mutational analysis of BRAF and K-ras in gastric cancers: absence of BRAF mutations in gastric cancers. Hum Genet 114:118–120
Russo A, Bazan V, Migliavacca M et al (2001) DNA aneuploidy and high proliferative activity but not K-ras-2 mutations as independent predictors of clinical outcome in operable gastric carcinoma: results of a 5-year Gruppo Oncologico dell’Italia Meridonale (GDIM) prospective study. Cancer 92:294–302
Yoo J, Park SY, Robinson RA, Kang SJ, Ahn WS, Kang CS (2002) ras Gene mutations and expression of Ras signal transduction mediators in gastric adenocarcinomas. Arch Pathol Lab Med 126:1096–1100
Hongyo T, Buzard GS, Palli D et al (1995) Mutations of the K-ras and p53 genes in gastric adenocarcinomas from a high-incidence region around Florence, Italy. Cancer Res 55:2665–2672
Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA (1998) Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst 90:675–684
Stella G, Rojas Llimpe FL, Barone C et al (2009) KRAS and BRAF mutational status and response to cetuximab combination therapy in advanced gastric cancer (GC) patients. In: Proceedings of American society of clinical oncology 2009 gastrointestinal cancers symposium, 15–17 Jan 2009 (abstr 34)
Acknowledgments
This study was supported by grant 0610520 from the Research Institute and Hospital, National Cancer Center, Goyang, Gyeonggi, Republic of Korea.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. R. Park and M.-C. Kook contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, S.R., Kook, MC., Choi, I.J. et al. Predictive factors for the efficacy of cetuximab plus chemotherapy as salvage therapy in metastatic gastric cancer patients. Cancer Chemother Pharmacol 65, 579–587 (2010). https://doi.org/10.1007/s00280-009-1067-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1067-9