Abstract
Purpose
Our objective was to assess the efficacy and toxicity of concurrent chemoradiotherapy with cisplatin + weekly divided-dose docetaxel in patients with stage III non-small-cell lung cancer (NSCLC).
Methods
A total of 34 patients aged less than 75 years old with locally advanced stage III NSCLC were enrolled. The patients received intravenous infusions of cisplatin (80 mg/m2; day 1) and docetaxel (20 mg/m2; days 1, 8, 15), followed by a week’s drug-free interval. Standard concurrent thoracic radiotherapy was given for 6 weeks (2 Gy per fraction; total dose, 60 Gy).
Results
Over Grade 3 neutropenia, esophagitis and pulmonary toxicities were observed in 23.5, 17.6 and 11.8% of the cases, respectively. One complete response and 20 partial responses were obtained, with an objective response rate of 61.8%. The median survival time was 26.4 months (95% CI 16.9—not reached) and the 1- and 3-year survival rates were 76.5 and 41.2%, respectively.
Conclusion
Cisplatin + weekly docetaxel with concurrent radiotherapy is a feasible and effective regimen for locally advanced NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locally advanced unresectable non-small-cell lung cancer (NSCLC), namely, stage IIIA and stage IIIB disease without pleural effusion, is one of the major targets of clinical research in the field of medical oncology. Thoracic radiotherapy (TRT) combined with chemotherapy is currently the standard medical treatment for locally advanced unresectable NSCLC [1–6]. In addition, recent clinical trials and a meta-analysis have suggested the superior effect of concurrent over sequential TRT with chemotherapy in patients with locally advanced NSCLC, although the available clinical data are insufficient [4–6]. For example, Furuse et al. [4] reported that concurrent TRT with cisplatin (CDDP), vindesine and mitomycin chemotherapy yielded a superior response rate and median survival time as compared with sequential radiotherapy with the same chemotherapeutic regimen. The median survival time was 16.5 months and the 2- and 3-year survival rates were 34.6 and 22.3%, respectively, in the concurrent chemoradiotherapy group.
Several novel agents have been introduced since the 1990s. Among these, docetaxel, a semithynthetic taxoid, has been shown to exhibit significant activity against NSCLC [7]. The clinical benefit of docetaxel administered in combination with CDDP has been evaluated in large trials and is currently one of the most effective treatments available for patients with NCSLC [8, 9]. In addition, treatment with docetaxel + CDDP has also been demonstrated to improve the survival of patients with metastatic NSCLC as compared to that with vindesine + CDDP [9].
Docetaxel has been shown to have a radiosensitizing effect owing to its effect of inducing cell cycle arrest in the most radiosensitive G2/M phase of the cell cycle [10, 11]. Based on these advantages, alternative therapeutic schedules, especially weekly administration of docetaxel with concurrent TRT has been studied in breast cancer [12] and NSCLC patients [13, 14], and encouraging, high response rates have been obtained. Koukourakis et al. [13] used docetaxel at 20–30 mg/m2 for 5 weeks and reported an overall response rate of 77% in NSCLC patients. Thus, weekly docetaxel combined with concurrent TRT is a valid treatment strategy and may improve the therapeutic outcomes in patients with NSCLC.
We showed in our previous phase I and II studies that treatment using CDDP (80 mg/m2; day 1) combined with weekly docetaxel (25 mg/m2; days 1, 8, 15) is feasible and associated with a low incidence of hemototoxicity, and is still effective in patients with advanced and metastatic NSCLC [15, 16]. CDDP has also been shown, both ex vivo and in vivo, to have a synergistic effect with radiation [17, 18]. Thus, we employed this chemotherapeutic regimen used by us previously, namely, CDDP + weekly docetaxel, for patients with locally advanced stage III NSCLC, who were scheduled to receive concurrent TRT. The primary objective of the present study was to determine the feasibility of administration and secondary objectives included the efficacy and overall survival of weekly docetaxel + CDDP with concurrent TRT in patients with locally advanced stage III NSCLC.
Materials and methods
Patient eligibility
Patients were eligible if they had histologically or cytologically proven unresectable locally advanced NSCLC (clinical stage III), with no history of prior therapy. If patients with stage IIIB disease had malignant pleural effusion or supraclavicular nodes, or the primary tumor size exceeded half of one lung, they were excluded. The other inclusion criteria were: (1) age, 20–75 years old, (2) Eastern Cooperative Oncology Group (ECOG) performance status (PS), 0 or 1 [19], (3) measurable disease and estimated life expectancy of over 3 months, (4) adequate bone marrow function (neutrophil count >2,000 μl−1, hemoglobin >10 g/dl, platelet count >100,000 μl−1), normal renal function (creatinine <1.5 mg/dl, creatinine clearance >60 ml/min), normal hepatic function (total bilirubin <1.5 mg/dl, serum aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels < twice the upper limit of the normal range), and normal pulmonary function [partial arterial oxygen tension (PaO2) >70 Torr, forced expiratory volume in 1 s >70%]. Patients were excluded from the trial if they had: (1) active infection, (2) severe heart disease, (3) past history of hypersensitivity to drugs, (4) pericardial effusion requiring drainage, (5) pregnancy, (6) an active double malignancy, (7) interstitial pneumonia as detected on a chest radiograph, or (8) an initial radiation field exceeding 50% of one lung. No other concomitant anticancer therapy or experimental drug administrations of any type was permitted. Written informed consent was obtained from each of the patients prior to their entry into the study.
Pretreatment evaluation and treatment protocol
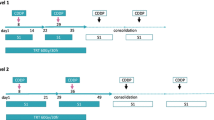
Before enrollment in the trial, all patients underwent a clinical evaluation namely, evaluation of the medical history, general condition, PS, hemogram including differential leukocyte count, routine laboratory tests, 24-h creatinine clearance, and urinalysis. Electrocardiography, chest radiography, pulmonary function testing, chest computed tomography (CT), abdominal ultrasound and/or CT and whole- brain CT or magnetic resonance imaging and isotope bone scan were performed in all the patients. The treatment schedule is summarized in Fig. 1. On day 1, CDDP (80 mg/m2) was dissolved in 500 ml of 0.9% saline and administered by intravenous infusion over a 3-h period after the docetaxel infusion. The drug was administered along with forced diuresis, which was accomplished by administration of at least 3,300 ml of i.v. fluids on days 1 and 2. Intravenous ondansetron (8 mg) was administered prophylactically and additional antiemetic treatment (prochlorperazine) was given as necessary for a further 5 days after the drug administration. Docetaxel (20 mg/m2) was diluted in 500 ml of 5% glucose and administered by intravenous infusion over a 1-h period on days 1, 8 and 15. Docetaxel was administered similarly on days 8 and 15 also, and no other fluids were given on the days of administration of docetaxel. Docetaxel was discontinued if the neutrophil count was less than 1,000 μl−1 or the platelet count was less than 75,000 μl−1 on days 8 and 15. The second cycle of chemotherapy was initiated on day 29. If the neutrophil count or platelet count was less than 2,000 or 100,000 μl−1, respectively, on day 28, the second cycle chemotherapy was delayed until recovery of the counts. The chemotherapy was continued for at least two cycles unless the patient experienced unacceptable toxicity or showed progression of the disease. The following therapy was optional and depended on investigator’s decision. TRT was begun concurrently on day 2 after the chemotherapy in all the patients. CT-based three- dimensional planning was conducted for treatment planning. Gross tumor volume (GTV) was defined based on the volume of the primary tumor and the involved nodes. The prescribed dose was 60 Gy, administered in 30 fractions over 6 weeks in each patient. Initial anterior–posterior opposed beams included the GTV with a 1–1.5 cm margin. The irradiation field was reduced to spare the spinal cord when the accumulated radiation dose to the spinal cord exceeded 40 Gy, and off-cord oblique beams were boosted up to 60 Gy according to the degree of shrinkage of the tumor and lymph nodes as estimated by subsequent CT.
Toxicity and response evaluation
During the study, complete blood cell counts including the differential cell count were obtained twice a week, or every 2 days in the case of Grade 3 or more severe neutropenia. Physical examinations and routine chemistry measurements were performed weekly during the treatment. If necessary, additional examinations of the blood counts were also performed. Toxicities were evaluated according to the National Cancer Institute’s Common Toxicity Criteria, version 2.0, and delayed radiation toxicities occurring more than 90 days after the start of chemoradiotherapy were assessed according to the Late Radiation Morbidity Scoring Scheme of the Radiation Therapy Oncology Group/Eurpean Organization for the Research and Treatment of Cancer [20]. Tumor assessment by chest CT was performed after two cycles of chemotherapy; the tumor response was evaluated according to the World Health Organization criteria [21]. All responses were carefully evaluated and confirmed by independent verification. Partial response (PR) was defined as a >50% decrease in the sum of the products of the longest perpendicular dimensions of all the measurable lesions for a period of 4 weeks. Progressive disease (PD) was defined as a 25% or greater increase in the tumor size, or the appearance of new lesions. Progression-free survival and overall survival were calculated from the date of initiation of the therapy to the time of detection of PD, death or the date of last follow-up (cutoff date: 31 December 2007). The Kaplan–Meier method was employed to determine the medians and 95% confidence intervals (CI) of the time-related parameters.
Results
Patient characteristics
Between April 1998 and March 2004, 34 patients were enrolled in the present study. The clinical characteristics of the patients are summarized in Table 1. The patients comprised 32 men and 2 women, with a median age of 61.4 years (range 45–75 years). Of the 34 patients, 30 had an ECOG PS score of 0, and 4 had a PS score of 1. The predominant histological type was adenocarcinoma (n = 19, 55.9%), followed in frequency by squamous cell carcinoma (n = 14, 41.2%); only one patient had large cell carcinoma. Three patients had stage IIIA disease and the remaining 31 patients had stage IIIB disease.
Toxicity
Toxicities were evaluated in all the patients. The number of patients entered at the highest grade level of each toxicity during the chemoradiotherapy is shown in Table 2a and b. The hematologic toxicities during the therapy were generally mild (Table 2a). Grade 3/4 leukopenia and neutropenia occurred in only 11.8 and 23.5% of the patients, respectively. None of the patients developed febrile neutropenia. Grade 3/4 thrombocytopenia was detected in three patients (8.8%); and the docetaxel administration on day 15 was skipped in these patients. The day-15 docetaxel had to be cancelled in both the first and second courses of treatment in one of these patients, and only in the second course of treatment in the remaining two cases. All the patients could be started on the second cycle of chemotherapy on day 29. Thus, there were no cases in which treatment delay of the second cycle of treatment was necessitated on account of any toxicity.
Nausea, vomiting and anorexia (Grade 1/2) were recorded in many cases, but they were well tolerated in all cases (Table 2b). There were no patients who complained of nausea and/or vomiting on the second (day 8) and third days (day 15) of docetaxel administration. One patient developed ischemic colitis during the first course of chemotherapy, and subsequent chemotherapy was discontinued. The hepatorenal toxicities were mild. Esophagitis more severe than Grade 3 was observed in 17.6% of the patients. Radiotherapy needed to be interrupted in one patient for one week because of the esophagitis, but this patient eventually received the entire radiation dose of 60 Gy. One patient developed a bronchoesophageal fistula months after the chemoradiotherapy. He was treated by double-stent implantation in both the esophagus and the left main bronchus. Grade 3/4 radiation pneumonitis occurred in four patients (11.8%), while Grade 1/2 radiation pneumonitis was noted in 44.1% of the patients.
Efficacy
Analysis of the response rate was performed in 34 patients. There was one complete response and 20 PRs, with an objective response rate of 61.8% (95% CI 45.0–76.1). Twelve patients were still disease-free as of December 2007 and one patient died of unrelated cause. Analysis of patterns of the first failure in 21 patients revealed local/regional-only disease in ten patients (47.6%), local/regional and distant disease in five patients (23.8%) and distant-only disease in six patients (28.6%). In the six cases of distant-only disease, only two showed that brain was first and the only site of relapse. In three patients who showed PR, surgical resection was judged to be feasible because of down-staging of the disease. Of these, two patients remained disease-free for 78.4 and 39.5 months, respectively, after the surgery. The third patient died 36 months after the surgery due to distant metastasis. The overall survival curve in all cases is shown in Fig. 2. The median survival time was 26.4 months (95% CI 16.9 months—not reached), and the 1-, 2- and 3-year survival rates were 76.5%, 52.9 and 41.2%, respectively. The median progression-free survival was 16 months (95% CI 5–47 months) and the 1- and 2-year progression-free survival rate was 52.9% (95% CI 36.2–69.7%) and 41.2% (95% CI 24.6–57.7%), respectively.
Discussion
The present study showed that weekly docetaxel (20 mg/m2, days 1, 8, 15) + CDDP (80 mg/m2; day 1) with concurrent TRT (60 Gy) is a feasible combined-modality treatment with moderate toxicity. Only one patient required rest from TRT due to esophagitis. Docetaxel administration needed to be skipped due to hematological toxicities in three patients and chemotherapy needed to be discontinued altogether after the first course due to the development of ischemic colitis. Thus, this concurrent chemoradiotherapy could be completed in a total of 29 (85.3%) patients without any modifications. Although Grade 3/4 esophageal and pulmonary toxicities were observed 17.6 and 11.8% of the patients, respectively, there were no patients with febrile neutropenia and/or who needed dose modification of the chemotherapy. Response to the therapy was observed in 61.8% of the patients, with a median survival time of 26.4 months and survival rates of 76.5% at 1 year and 41.2% at 3 years.
Phase I/II trials of other modified dosing schedules of CDDP + docetaxel with concomitant TRT in patients with unresectable stage III NSCLC have been reported [22–26]. Yamamoto et al. [22] conducted a dose escalation study of weekly CDDP + docetaxel with concurrent TRT for NSCLC and recommended administration of CDDP at 25 mg/m2 and docetaxel at 20 mg/m2 on days 1, 8 and 15, with the cycles repeated every 4 weeks. Wu et al. [23] also conducted a trial using the same schedule of chemotherapy and reported the recommended dose of 20 mg/m2 for CDDP and of 20 mg/m2 for docetaxel administered each week for 6 weeks, respectively. Furthermore, in a dose escalation study with weekly docetaxel alone and concurrent radiotherapy for patients with NSCLC, docetaxel administration at 20 mg/(m2 week) was recommended [13]. Based on these results and our previous studies [15, 16], we selected the docetaxel dose of 20 mg/m2 in the present study. Although there was no dose escalation trial in the present study, we thought that the weekly dose of docetaxel (20 mg/m2) was extremely well-tolerated, with low incidences, in particular, of Grade 3/4 neutropenia and neutropenic infections, even when the drug was administered in combination with CDDP (80 mg/m2) and TRT at 60 Gy.
A high incidence of esophageal and pulmonary toxicities has been reported with concurrent chemoradiotherapy [3, 4, 6, 13, 14]. For the CDDP + docetaxel regimen, the frequency of severe esophageal toxicity was reported to occur at an incidence of 8–25% [22, 25, 26]. The esophageal toxicity was found to be modest in the present study, except in one patient who developed a broncho-esophageal fistula 3 months after the completion of chemoradiotherapy. The primary lesion in this patient was located in left S6 and had been found to extend to the esophagus even before the therapy. Since there was no evidence of relapse in the lesion, the fistula seemed to represent a therapy-related toxicity. Severe pulmonary toxicity, including in the late phase was observed in four cases (11.8%) in the present study. The frequency of severe pulmonary toxicity seemed to be identical to that reported for other chemotherapeutic agent combinations (8–20%) [3, 27, 28], but higher than that reported for modified CDDP + docetaxel regimens (0–4.8%) [22–26]. Onishi et al. [14] suggested an increased risk of radiation pneumonitis associated with weekly docetaxel combined with TRT for stage III NSCLC, because they observed pneumonitis greater than Grade 3 in severity pneumonitis in 47% cases. Thus, the precise risk of radiation pneumonitis associated with divided-dose docetaxel administration remains unresolved. Further close examination is needed to reach a definitive conclusion.
Asthenia was also one of the dose-limiting toxicities of weekly divided-dose administration of docetaxel [12–14, 29, 30]. Asthenia was a cumulative toxicity, resulting in the treatment refusal or dose reduction. Hainsworth et al. [29] reported that fatigue as an adverse effect of weekly docetaxel was more severe in elderly patients than in younger patients. In addition, Ohe et al. [30] reported that the severity of fatigue with weekly docetaxel administration increased with increase in the weekly dose of docetaxel. In the present study, therapy-related fatigue and asthenia were only mild. This may reflect the fact that the dose of 20 mg/m2 per course, the cumulative dose and the age of the enrolled patients were relatively low in our trial as compared with those in other studies [29, 30]. We think that weekly docetaxel at the dose of 20 mg/m2 was well-tolerated in terms of the incidence and severity of treatment-related fatigue.
In the present study, the overall response rate to the combined-modality therapy was 61.8%, and the 2-year survival rate was 52.9%. Although the response rate seemed slightly disappointing, we achieved 2- and 3-year survival rates of 52.9 and 41.2%, respectively. Kiura et al. [25] also studied biweekly administration of both agents docetaxel + CDDP with concurrent TRT and reported a median survival time of 23.4 months, with an overall survival rate of 76% at 1 year and 54% at 2 years, the results being nearly identical to ours. The trial was also well tolerated. Survival in any trial has to be balanced against toxicity and compliance [31]. The current trial provided satisfactory prolongation of the survival rates, perhaps on account of the reduced toxicity of and enhanced compliance with the treatment schedule.
In recently published studies of concurrent chemoradiotherapy, the median survival time obtained with divided-dose chemotherapy was 23 months (vinorelbine/CDDP, both administered in divided doses on days 1 and 8 every 3 weeks) [32] or 27 months (biweekly docetaxel/carboplatin) [33]. These findings, included the findings in our present study, suggest that the efficacy of modified and divided-dose schedules of administration of chemotherapeutic agents is superior to that of conventional administration schedules [3, 4, 6] in combined-modality therapy, e.g., platinum- containing chemotherapy and concurrent TRT. However, the optimum chemotherapeutic regimen using newer chemotherapeutic agents for combination with radiotherapy has not yet been established and there are no data available from phase III studies. Among the newly developed agents, weekly administration of docetaxel could be considered as one of the possibilities for locally advanced stage III NSCLC.
In summary, our results indicate that weekly docetaxel (20 mg/m2, days 1, 8, 15) plus cisplatin (80 mg/m2, day 1) with concurrent thoracic irradiation (60 Gy) is a potentially feasible combined-modality treatment, with moderate toxicity. The treatment regimen appears to be promising and merits further evaluation in patients with stage III NSCLC.
References
Dillman RO, Herndon J, Seagren SL et al (1996) Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 88:1210–1215
Sause W, Kolesar P, Taylor SIV et al (2000) Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 117:358–364
Belani CP, Choy H, Bonomi P et al (2005) Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 23:5883–5891
Furuse K, Fukuoka M, Kawahara M et al (1999) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17:2692–2699
Curran D, Scott C, Langer C et al (2003) Long-term benefit is observed in a phase III comparison of sequential versus concurrent chemo-radiotherapy for patients with unresected stage III NSCLC: RTOG 9410. Proc Am Soc Clin Oncol 22:621
Fournel P, Robinet G, Thomas P et al (2005) Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95–01 Study. J Clin Oncol 23:5910–5917
Kunitoh H, Watanabe K, Furuse K et al (1996) Phase II trial of Decetaxel in previously untreated advanced non-small–cell lung cancer: a Japanese Cooperative study. J Clin Oncol 14:1649–1655
Schiller JH, Harrington D, Belani CP et al (2002) Eastern Cooperative Oncology Group. For the Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Kubota K, Watanabe K, Kunitoh H et al (2004) Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol 22:254–261
Choy H, Rodriguez S, Koester S et al (1993) Synergic effects of Taxol/Taxotere on radiation sensitivity on human tumor cell lines. Int J Radiat Oncol Biol Phys 24:274–275
Mason KA, Hunter NR, Milas M et al (1997) Docetaxel enhances tumor radioresponse in vivo. Clin Cancer Res 3(12 Pt 1):2431–2438
Karasawa K, Katsui K, Seki K et al (2003) Radiotherapy with concurrent docetaxel for advanced and recurrent breast cancer. Breast Cancer 10:268–274
Koukourakis MI, Kourousis C, Kamilaki M et al (1998) Weekly docetaxel and concomitant boost radiotherapy for non-small cell lung cancer. A phase I/II dose escalation. Eur J Cancer 34:838–844
Onishi H, Kuriyama K, Yamaguchi M et al (2003) Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: a good local response but no gut survival due to radiation pneumonitis. Lung Cancer 40:79–84
Koizumi T, Tsunoda T, Fujimoto K et al (2001) Phase I trial of weekly docetaxel combined with cisplatin in patients with non-small-cell lung cancer. Lung Cancer 34:125–131
Tsunoda T, Koizumi T, Hayasaka M et al (2004) Phase II study of weekly docetaxel combined with cisplatin in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 54:173–177
Alvarez MV, Cobreros G, Heras A et al (1978) Studies on cis-dichlorodiammineplatinum (II) as a radiosensitizer. Br J Cancer 3:68–72
Dewit L (1987) Combined treatment of radiation and cisdiamminedichloroplatinum (II): a review of experimental and clinical data. Int J Radiat Oncol Biol Phys 13:403–426
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and Response Criteria Of The Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
National Cancer Institute: Common Toxicity Criteria (CTC) v 2.0. http://ctep.cancer.gov/reporting/ctc.html
Miller AB, Hoogstraten B, Staquet M et al (1981) Reporting results of cancer treatment. Cancer 47:207–214
Yamamoto N, Nishimura Y, Nakagawa K et al (2006) Phase I/II study of weekly docetaxel dose escalation in combination with fixed weekly cisplatin and concurrent thoracic radiotherapy in locally advanced non-small cell lung cancer. Cancer Chemother Pharmacol 58:285–291
Wu HG, Bang YJ, Choi EK et al (2002) Phase I study of weekly docetaxel and cisplatin concurrent with thoracic radiotherapy in Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 52:75–80
Mudad R, Ramsey M, Kovitz K et al (2003) Concomitant weekly docetaxel, cisplatin and radiotherapy in locally advanced non-small cell lung cancer: a dose finding study. Lung Cancer 39:173–177
Kiura K, Ueoka H, Segawa Y et al (2003) Phase I/II study of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer. Br J Cancer 89:795–802
Iwasaki Y, Ohsugi S, Natsuhara A et al (2006) Phase I/II trial of biweekly docetaxel and cisplatin with concurrent thoracic radiation for stage III non-small-cell lung cancer. Cancer Chemother Pharmacol 58:735–741
Albain KS, Crowley JJ, Turrisi AT 3rd et al (2002) A Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol 20:3454–3460
Robert F, Spencer SA, Childs HA 3rd et al (2002) Concurrent chemoradiation therapy with cisplatin and paclitaxel for locally advanced non-small cell lung cancer: long-term follow-up of a phase I trial. Lung Cancer 37:189–199
Hainsworth JD, Burris HA 3rd, Erland JB et al (1998) Phase I trial of docetaxel administered by weekly infusion in patients with advanced refractory cancer. J Clin Oncol 16:2164–2168
Ohe Y, Niho S, Kakinuma R et al (2001) Phase I studies of cisplatin and docetaxel administration by three consecutive weekly infusions for advanced non-small cell lung cancer in elderly and non-elderly patients. Jpn J Clin Oncol 31:100–106
Socinski MA, Zhang C, Herndon JE 2nd et al (2004) Combined modality trials of the Cancer and Leukemia Group B in stage III non-small-cell lung cancer: analysis of factors influencing survival and toxicity. Ann Oncol 15:1033–1041
Hirose T, Mizutani Y, Ohmori T et al (2006) The combination of cisplatin and vinorelbine with concurrent thoracic radiation therapy for locally advanced stage IIIA or IIIB non-small-cell lung cancer. Cancer Chemother Pharmacol 58:361–367
Sakai H, Yoneda S, Kobayashi K et al (2004) Phase II study of bi-weekly docetaxel and carboplatin with concurrent thoracic radiation therapy followed by consolidation chemotherapy with docetaxel plus carboplatin for stage III unresectable non-small cell lung cancer. Lung Cancer 43:195–201
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakamura, M., Koizumi, T., Hayasaka, M. et al. Cisplatin and weekly docetaxel with concurrent thoracic radiotherapy for locally advanced stage III non-small-cell lung cancer. Cancer Chemother Pharmacol 63, 1091–1096 (2009). https://doi.org/10.1007/s00280-008-0837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0837-0