Abstract
Purpose
A pharmacologic analysis of intracavitary doxorubicin in the treatment of patients with intracavitary cancer dissemination was performed to further evaluate the possible benefits of this treatment modality.
Methods
Twenty appendiceal malignancy patients with peritoneal carcinomatosis (PC), three appendiceal malignancy patients with direct extension into the pleural cavity, 20 patients with peritoneal mesothelioma and one patient with pleural mesothelioma were available for pharmacologic monitoring. After intraperitoneal or intrapleural administration of doxorubicin, plasma and peritoneal fluid samples were obtained at 15, 30, 45, 60 and 90 min in all patients. After intrapleural administration, plasma and pleural fluid samples were collected at similar intervals. Tumor and normal tissues were obtained when available. Doxorubicin concentrations were determined by high-performance liquid chromatography (HPLC).
Results
Intraperitoneal doxorubicin showed a prolonged retention in the peritoneal cavity. Doxorubicin concentrations in tumor tissue were consistently elevated above intraperitoneal concentrations from 30 through 90 min. For appendiceal malignancy, the concentrations of doxorubicin were significantly higher in minimally aggressive mucinous tumors. Pleural chemotherapy solutions retained doxorubicin to a greater extent than peritoneal fluid.
Conclusions
Doxorubicin shows characteristics favorable for intracavitary administration with sequestration of doxorubicin in cancer nodules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritoneal carcinomatosis (PC) is a common manifestation of intraabdominal malignancies. In patients with peritoneal mesothelioma, the peritoneal surface malignancy is a primary disease. In most patients, however, it is the result of transcoelomic spread of a primary tumor into the peritoneal cavity. Tumor emboli can disperse throughout the peritoneal cavity preoperatively as the direct result of transserosal invasion or rupture of the primary tumor. Alternatively, intraperitoneal spread may be induced iatrogenically at the time of surgery by tumor cells escaping from transected bowel, lymph vessels or blood vessels. The tumor cell entrapment hypothesis suggests that these cancer emboli readily implant on the raw surgical tissue surfaces where they are under the growth-stimulating influence of inflammatory cells and growth factors [1, 2]. At the same time, the postoperative fibrin deposits accumulated at wounded sites isolate the tumor cells from the host defenses. The high incidence of cancer cell implantation on peritoneal surfaces is in great contrast to the relative metastatic inefficiency for cancer cells implanting within the vascular system as systemic metastases [3].
In the past oncologists have assumed that PC is equal to distant metastases and as such regarded it as an incurable component of intraabdominal malignancy. However, over the last two decades novel therapeutic approaches have emerged for patients with isolated peritoneal metastases of gastrointestinal cancer, ovarian cancer and primary peritoneal malignancies. The underlying revised hypothesis considers PC as a loco-regional disease that may have regional treatment options. A combined approach uses both surgery and perioperative chemotherapy. First, an aggressive surgical cytoreduction utilizes visceral resections and peritonectomy procedures to remove all gross and macroscopic disease. Then perioperative intraperitoneal chemotherapy is aimed at the residual microscopic disease [4]. The perioperative intraperitoneal chemotherapy includes Hyperthermic Intraperitoneal Chemotherapy (HIPEC) and/or Early Postoperative Intraperitoneal Chemotherapy (EPIC).
This shift in the treatment paradigm has resulted in encouraging clinical results as contrasted to prior failures. Several phase III-trials have reported a survival and progression-free survival benefit of intraperitoneal chemotherapy [5–8].
The selection of chemotherapeutic regimen used in perioperative chemotherapy protocols has been based on research of chemotherapeutic responses in systemic administration and on pharmacodynamic and pharmacokinetic properties of the drugs following intraperitoneal administration. Although this will change in the future, to this point in time drug selection has not been based on specific clinical or pharmacological trials that compare the survival benefits of different intraperitoneal treatment regimen.
Because of its wide in vitro and in vivo activity against a broad range of malignancies, its slow clearance from the peritoneal compartment due to the high molecular weight of the hydrochloride salt (57,999 Dalton) and the absence of risk for dose-limiting cardiotoxicity when used intraperitoneally, doxorubicin was considered a potential beneficial agent for perioperative intraperitoneal delivery. The aim of this study was to critically analyze our current pharmacologic data regarding the use of intraperitoneal doxorubicin in the treatment of peritoneal surface malignancies.
Materials and methods
At the Washinton Cancer Institute 20 patients with peritoneal carcinomatosis from appendiceal malignancy, three patients with direct extension of appendiceal malignancy into the pleural cavity, 20 patients with peritoneal mesothelioma and one patient with pleural mesothelioma were included in this study. Ethical approval for the study was obtained. After completion of cytoreductive surgery, patients were treated with HIPEC using the technique as described by Sugarbaker et al. [9]. The doxorubicin regimen used for both malignancies was 15 mg/m2 in 1.5 L/m2 of 1.5% dextrose peritoneal dialysis solution administered over 90 min at approximately 42.5°C. Hyperthermic conditions were maintained by recirculating chemotherapy solution through a heat exchanger at 45°C. Prior to treatment a 3-mL reference sample of the chemotherapy solution was obtained along with a 3-mL sample of blood and urine. At 15, 30, 45, 60 and 90 min during the 90-min HIPEC treatment, 3-mL samples of peritoneal fluid, blood and urine were obtained for high-performance liquid chromatography (HPLC) determination of doxorubicin concentrations. In some patients small tumor nodules (0.5–2 cm) were present on the small bowel or small bowel mesenteries which were marked by a suture during cytoreduction. These nodules were harvested at the same time intervals during the HIPEC using a curved Mayo scissors. In an occasional patient a portion of normal small bowel that required resection was present from which samples of normal peritoneal tissue were resected during HIPEC. Urine output was monitored at 15-min intervals throughout the procedure. A diuresis of greater than 400 mL per hour was maintained. At 90 min all peritoneal fluid was drained and the volume was recorded. A tumor sample was obtained at 120 min (30 min after complete drainage of peritoneal fluid) from a single patient in each group.
Processing and storage of samples
Blood and peritoneal fluid samples were centrifuged at 3,000 rpm for 10 min. The resulting plasma and supernatant from peritoneal fluid samples along with urine samples were stored in capped polypropylene tubes at −20°C. Tissue samples were dried of all surface moisture using absorbent gauze pads and stored at −20°C in capped polypropylene vials. HPLC assay of doxorubicin levels was performed within 48 h of collection.
Preparation of samples for HPLC analysis
All samples were thawed at room temperature before HPLC analysis. Peritoneal fluid and urine samples were diluted appropriately with methanol. After thorough mixing the resulting solutions were filtered through 0.45-µm syringe filters prior to HPLC injection.
For plasma samples, a 500 μL sample was mixed with 10 volumes of chloroform-isopropanol (2:1) in 15-mL screw-capped polypropylene centrifuge tubes. After thorough mixing followed by centrifugation the lower organic phase was transferred to a clean polypropylene centrifuge tube and evaporated to dryness under a stream of N2 at 37°C. The residue was dissolved in 250 μL of methanol and filtered through a 0.45-µm syringe filter prior to HPLC injection.
Tumor nodules and normal tissue samples were thawed and again dried of surface moisture before processing. Each sample was then weighed and homogenized in approximately 10 volumes of chloroform-isopropanol (2:1). The homogenate was transferred to a 15-mL polypropylene centrifuge tube and centrifuged at 3,000 rpm for 10 min. The supernatant was transferred to a clean polypropylene tube and evaporated with a N2 stream (as with plasma). The residue was dissolved in methanol (as required), filtered and 50 μL was injected for HPLC analysis. Daunorubicin (DNR) was used as an internal standard [10].
HPLC system
Doxorubicin concentrations were determined using a modified version of the HPLC method as described by Cummings et al. [10]. Briefly, the HPLC system consisted of a Shimadzu LC7A instrument equipped with an SPD-6AV (UV-VIS) detector set at 295 nm and a C-R6a ‘Chromatopac’ data processor (Shimadzu Instruments, Columbia, MD, USA). Chromatographic separation was accomplished on a C18 reversed phase column (Varian Associates, Walnut Creek, CA, USA). The mobile phase consisted of 28% acetonitrile in 0.1% orthophosphoric acid with 0.1% triethylamine. The flow rate was 1.2 mL/min and the sample injection volume was 50 μL.
Histologic classification of tumor tissues
Histologic character of appendiceal malignancy tissues was determined using the criteria as described by Ronnett and colleagues [11]. The appendiceal malignancy patients were separated into two groups. The patients whose histology was scored as disseminated peritoneal adenomucinosis (DPAM) showed a single layer of noninvasive bland appearing tumor cells surrounding copious mucus. Patients with mucinous peritoneal carcinomatosis (PMCA) had invasive peritoneal lesions composed of abundant glandular tissue with cytologic atypia.
The biological aggressiveness of peritoneal mesothelioma was estimated by a histologic assessment of nuclear size as described by Cerruto and colleagues [12]. The nuclear size of group I (10–20 μm) and group II (21–30 μm) was placed together as a non-aggressive histologic type and group III (31–40 μm) and group IV (>40 μm) placed together as an aggressive histologic type.
Data presentation and statistical analysis
Concentration times time graphs were constructed for these data. The mean ± one standard deviation was shown. The AUC for doxorubicin in plasma, peritoneal fluid or tumor tissue were obtained from GraphPad Prism analyses. A statistical comparison of continuous variables was performed using a Student’s t test. Significance was accepted with a P value of less than 0.05.
Results
Patient characteristics and pathologic information for 20 patients with pseudomyxoma peritonei and 20 patients with peritoneal mesothelioma who had intraperitoneal doxorubicin administration are summarized in Table 1. The three patients with direct extension of appendiceal malignancy into the pleural space and the one patient with pleural mesothelioma are not included.
Doxorubicin concentrations in plasma, peritoneal fluid, tumor nodules and normal tissue
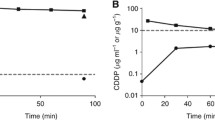
As an example, the data obtained in these patients is shown in Fig. 1. In a single patient, plasma and peritoneal fluid concentrations of doxorubicin over the 90 min treatment were available. Also five tumor nodules on small bowel or its mesentery were available and five pieces of normal peritoneal surface from the small bowel were obtained. Over the 90 min 93% of the doxorubicin was cleared from the peritoneal cavity. Plasma concentrations and normal tissue concentrations peaked at 15 min; tumor tissue concentrations peaked at 90 min. AUC ratio of peritoneal fluid to plasma was 73, of tumor to plasma was 128 and of tumor tissue to peritoneal fluid was 1.8. The concentration of doxorubicin in tumor nodules and in normal tissues was consistently larger than in intraperitoneal fluid after 15 min.
Doxorubicin pharmacokinetics compared in appendiceal malignancy patients to mesothelioma patients
Plasma and peritoneal fluid concentrations were obtained in 20 appendiceal malignancy patients and 20 peritoneal mesothelioma patients and the data shown in Fig. 2. For both groups of patients taken together the AUC ratio peritoneal fluid to plasma was 90. A statistical analysis of the pharmacologic comparisons of doxorubicin in these two diseases is presented in Table 2. There was a statistically significant difference in the AUC peritoneal fluid to plasma ratio for these two diseases (P = 0.004).
Doxorubicin pharmacokinetics compared in patients with two histologic types of appendiceal malignancy and in two histological types of peritoneal mesothelioma
In the patients with appendiceal malignancy, 8 had DPAM and 12 had PMCA. Figure 3 shows the peritoneal fluid and tumor tissue doxorubicin concentrations in patients with these two histological types of tumor. The peritoneal fluid concentrations were the same; the concentrations of doxorubicin in DPAM tumor nodules were statistically significantly elevated (P = 0.02) when compared to PMCA tumor nodules (P = 0.02). When the peritoneal fluid and tumor tissue doxorubicin concentrations in patients with a non-aggressive histologic type of peritoneal mesothelioma were compared to the aggressive histologic type no significant differences were observed.
Doxorubicin pharmacokinetics compared in patients with intrapleural versus intraperitoneal chemotherapy administration
Four patients had the intrapleural administration of doxorubicin and 40 had intraperitoneal administration. The pharmacokinetics of intrapleural fluid versus intraperitoneal fluid were markedly different with a slower clearance of this drug from the pleural space (P = 0.008). Plasma concentrations were similar with a trend toward increased plasma levels at 90 min with pleural doxorubicin administration (Fig. 4).
Discussion
We provide new pharmacologic data supporting the use of intracavitary doxorubicin in the treatment of patients with PC.
Mechanism of action of doxorubicin
Doxorubicin (C27H29NO11) or hydroxyldaunorubicin (adriamycin) is an anthracycline antibiotic used in the treatment of a wide range of cancers. Historically it has been categorized as a DNA-intercalating drug although the exact mechanism of action is complex and still unclear. Most authors today agree that the process initiated by this class of drugs is a series of coupled events (rather than several parallel actions) that involves both membrane and nuclear components, but which are part of a single ordered mechanism of action which may be augmented by hyperthermia [13, 14]. Lane and co-workers [15] provided evidence that a phase-change in the cell surface membrane occurs at the same temperature (20°C) as the loss of biological activity of doxorubicin. They state that the interaction of doxorubicin with the cell surface membrane rather than its intracellular uptake is an essential first step for doxorubicin cytotoxity.
Rationale for intracavitary administration
Doxorubicin has shown activity in a wide variety of intraabdominal malignancies such as ovarian carcinoma, gastric carcinoma, pancreas carcinoma, sarcomas, mesotheliomas and appendiceal malignancies [16]. However, in many clinical settings, systemically administered doxorubicin does not provide an adequate cytotoxic effect on peritoneal tumor nodules. With intracavitary administration a high level of the drug can be achieved in the peritoneal or pleural cavity with low systemic exposure. Johansen in 1981 [17] provided experimental animal data supporting substantial pharmacokinetic advantage for intraperitoneal administration following single doses of doxorubicin in nude mice compared to mice with intravenous administration. Doxorubicin was one of the first drugs extensively used in intraperitoneal cancer chemotherapy. Ozols and co-authors in 1982 [18] published a phase-I study of doxorubicin administered intraperitoneally in 10 patients with advanced ovarian cancer. This clinical study was following earlier experimental work in murine ovarian cancer and with human ovarian cancer cells [19–21]. This report revealed a dose-limiting toxicity of intraperitoneal doxorubicin by sclerosing peritonitis with a dose of 18 μM or greater. At the same time, three partial responses were observed in patients with small volume disease. Sugarbaker and colleagues [22] suggested a total dose of 15 mg/m2 in 3 L of 1.5% dextrose caused tolerable local sclerosis with normal bowel function. Roboz et al. in 1981 [23] reported a complete response after two intraperitoneal instillations of doxorubicin in a patient with recurrent ovarian cancer after previous surgery. The patient was free of disease 3 years after the procedure. Since these initial clinical reports the clinical experience with intraperitoneal doxorubicin has expanded substantially. These reports have suggested a clinical benefit of intraperitoneal doxorubicin in peritoneal mesothelioma, ovarian carcinoma, pseudomyxoma peritonei and a variety of other intraabdominal malignancies [24–26].
Our pharmacologic data provide some new information on the intracavitary use of doxorubicin in patients with peritoneal surface or pleural malignancy originating from appendiceal malignancy or peritoneal mesothelioma.
Intrapleural versus intraperitoneal pharmacokinetics
Our data suggest a different pharmacodynamic profile of intracavitary doxorubicin within the pleural cavity as compared to the peritoneal cavity. An augmented dose-intensification with an increased area under the curve is observed in the pleural cavity as compared to the peritoneal cavity. As far as is known the mesothelial lining in the two compartments is identical. There may be a number of possible explanations for these differences. First a difference in the organization of the submesothelial space is a possible explanation for this observed difference. Not the mesothelial lining but the blood capillary wall and the surrounding interstitial matrix are the principal barrier for clearance of molecules from the abdominal cavity [27]. Flessner et al. demonstrated in a rodent model that neither removal of the stagnant fluid layer on the mesothelium nor removal of the mesothelial lining itself influenced the mass transfer coefficient over the peritoneal barrier [28]. Ultrastructural comparison of the submesothelial space in pleura and peritoneum may reveal relevant differences supporting our clinical data.
Another explanation may be the different total diffusion surface of the pleural surface and the peritoneal surface [29]. The pleural space has a reduced total diffusion surface as compared to that provided by the extensive surface of the visceral peritoneum. Also, less blood flow through a lung partially collapsed to provide space within the pleural cavity for chemotherapy solution may retard the clearance of doxorubicin from pleural space to plasma.
Doxorubicin sequestration in tumor nodules
Contrary to intuitive thinking, the peritoneal concentration of a drug may not reflect the true drug levels in tumor nodules. Figures 1–3 show an elevated doxorubicin level in tumor nodules as compared to peritoneal fluid. The penetration of cytotoxic drugs into peritoneal tumor nodules is a complex process which is influenced by pharmacokinetic parameters including dose, concentration and exposure time of a cancer chemotherapy drug. Also factors as tumor vasculature, density of the tumor nodules, and size of the nodules are relevant [29]. Although a vast amount of literature has been published on the pharmacokinetic properties of cancer chemotherapy drugs, the available data on tumor tissue distribution of the drugs and thus efficacy is remarkably limited. Our data show the importance of such measurements. The pharmacodynamic profile of doxorubicin shows an unexpected sequestration of the drug in the tumor nodules. This finding is a consistent one in our study, regardless the underlying malignancy producing the peritoneal carcinomatosis or the histopathological grade of the disease. To our knowledge, such cancer chemotherapy drug accumulation in tumor nodules has not been previously reported in literature.
This finding has several consequences. First, the classical simplified two-compartment model as proposed by Dedrick and Flessner [29] is inadequate in terms of quantifying the accurate cancer chemotherapy drug levels in tumor nodules if one accepts that simple diffusion and not active transport is the main mechanism for the measured pharmacodynamic profile. We propose a four-compartment model involving plasma space, peritoneal space, subperitoneal space and tumor nodule. Preloading of the subperitoneal compartment with the cancer chemotherapy drug is the diffusion force responsible for the drug concentration measured in the tumor nodules. The sequestration phenomenon of doxorubicin in tumor nodules is a constant one in its presence regardless of the underlying pathology or subtype. The differences observed in the magnitude of sequestration for the different subtypes of appendiceal malignancy are probably due to differences in the physical characteristics (density) of the tumor nodules in DPAM and PMCA.
A second consequence is that the cancer chemotherapy levels measured in the tumor nodules may be more important than considered in the past. There are remarkable differences between the pharmacodynamic profiles of doxorubicin, and melphalan [30]. Our data show a consistent dose-intensification between peritoneal compartment and plasma compartment for each of these cancer chemotherapy drugs. However, doxorubicin is concentrated in tumor nodules; in constrast, melphalan concentration is reduced in tumor nodules using an identical assay methodology when compared to peritoneal drug levels.
The consistent finding of doxorubicin sequestration in tumor nodules raises questions about the possible underlying mechanism. Simple diffusion, forces as proposed by Dedrick and Flessner are not enough to explain the phenomenon. One possible explanation would be active transport. This, however, seems unlikely due to the fact that the less cellular DPAM takes up more doxorubicin than PMCA. Another possibility is irreversible binding of doxorubicin at the level of the tumor nodule. Whether this binding takes place at the individual cancer cell membrane as suggested by some authors [14–16] or at the level of the tumor nodule interstitium should be the subject of further research.
The relevance of these observed differences in chemotherapy sequestration at the level of the tumor nodules for the cytotoxic effects of these cancer chemotherapy drugs after intraperitoneal administration still needs to be established.
Conclusions
We present the pharmacologic data of intracavitary doxorubicin in patients with PC from appendiceal malignancy, peritoneal mesothelioma or pleural mesothelioma. Our data provide support for a revision of the mathematical diffusion model as proposed by Dedrick and Flessner [29]. A third and fourth compartment, the subperitoneal space and tumor nodule emerge as important new elements in the pharmacodynamic profile of each drug. These data suggest different cancer chemotherapy levels measured within the tumor nodules for different drugs. The true importance of these drug levels measured in the tumor nodules for the cytotoxic efficacy needs to be established.
References
Zoetmulder FA (1982) Modelstudies over het colorectale carcinoom. Amsterdam, Rodopi
Sugarbaker PH, Cunliffe WJ, Belliveau J et al (1989) Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol 16(4):83–97
Weiss L (1990) Metastatic inefficiency. Adv Cancer Res 54:159–211
Sugarbaker PH (2003) Peritonectomy procedures. Surg Oncol Clin N Am 12(3):703–727
Alberts DS, Liu PY, Hannigan EV et al (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335(6):1950–1955
Markman M, Bundy BN, Alberts DS et al (2001) Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Oncology Group. J Clin Oncol 19(4):1001–1007
Armstrong DK, Bundy B, Wenzel L et al (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354(1):34–43
Verwaal VJ, Van Ruth S, De Bree E et al (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21(20):3737–3743
Sugarbaker PH (2005) An instrument to provide containment of intraoperative intraperitoneal chemotherapy with optimized distribution. J Surg Oncol 92(2):142–146
Cummings J, Stuart JFB, Calman KC (1984) Determination of adriamycin, adriamycinol and their 7-deoxyaclycones in human serum by high-performance liquid chromatography. J Chromatogr 311:125–133
Ronnett BM, Zahn CM, Kurman RJ et al (1995) Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis: a clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol 19(12):1390–1408
Cerruto CA, Brun EA, Chang D, Sugarbaker PH (2006) Prognostic significance of histomorphologic parameters in diffuse malignant peritoneal mesothelioma. Arch Pathol Lab Med 130(11):1654–1661
Tritton TR (1991) Cell surface actions of adriamycin. Pharmacol Ther 49:293–309
Hahn GM, Braun J, Har-Kedar I (1975) Thermochemotherapy: synergism between hyperthermia (42–43°) and adriamycin (or bleomycin) in mammalian cell inactivation. Proc Nat Acad Sci 72(3):937–940
Lane P, Vichi P, Bain DL et al (1987) Temperature dependence studies of adriamycin uptake and cytotoxocity. Cancer Res 42:4038–4042
Carter SK (1981) Adriamycin—a review. J Natl Cancer Inst 55:1265–1274
Johansen PB (1981) Doxorubicin pharmacokinetics after intravenous and intraperitoneal administration in the nude mouse. Cancer Chemother Pharmacol 5:267–270
Ozols RF, Young RC, Speyer JL et al (1982) Phase I and pharmacological studies of adriamycin administered intraperitoneally to patients with ovarian cancer. Cancer Res 42:4265–4269
Ozols RF, Grotzinger KR, Fisher RI et al (1979) Kinetic characterization and response to chemotherapy in a transplantable murine ovarian cancer. Cancer Res 39:3202–3208
Ozols RF, Locker GY, Doroshow JH et al (1979) Pharmacokinetics and tissue penetration in murine ovarian cancer. Cancer Res 39:3209–3214
Ozols RF, Willson JKV, Weltz MD et al (1980) Inhibition of human ovarian cancer colony formation by adriamycin and its major metabolites. Cancer Res 40:4109–4112
Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D (2005) Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist 10(2):112–122
Roboz J, Jacobs AJ, Holland JF et al (1981) Intraperitoneal infusion of doxorubicin in the treatment of gynecologic carcinomas. Med Pediatr Oncol 9:245–250
Yan TD, Welch L, Black D et al (2007) A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 18(5):827–834
Raspagliesi F, Kusamura S, Campos Torres JC et al (2006) Cytoreduction combined with intraperitoneal hyperthermic perfusion chemotherapy in advanced/recurrent ovarian cancer patients: the experience of National Cancer Institute of Milan. Eur J Surg Oncol 32(6):671–675
Yan TD, Black D, Savady R et al (2007) A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol 14(2):484–492
Stelin G, Rippe B (1990) A phenomenological interpretation of the variation in dialysate volume with dwell time in CAPD. Kidney Int 38(3):465–472
Ceelen WP, Påhlman L, Mahteme H (2006) Pharmacodynamic aspects of intraperitoneal cytotoxic therapy. In Peritoneal carcinomatosis: a multidisciplinary approach. Cancer Treat Res, Springer, 195–214
Dedrick RL, Flessner MF (1997) Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst 89(7):480–487
Sugarbaker PH, Stuart OA (2007) Pharmacokinetic and phase II study of heated intraoperative intraperitoneal melphalan. Cancer Chemother Pharmacol 59(2):151–155
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van der Speeten, K., Stuart, O.A., Mahteme, H. et al. A pharmacologic analysis of intraoperative intracavitary cancer chemotherapy with doxorubicin. Cancer Chemother Pharmacol 63, 799–805 (2009). https://doi.org/10.1007/s00280-008-0800-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0800-0