Abstract
Objective
To evaluate the pharmacokinetic inter-patient variability of 30-min hyperthermic intraperitoneal oxaliplatin chemotherapy.
Patients and methods
Data were obtained from 24 patients who were treated according to two procedures of heated intra-operative intraperitoneal oxaliplatin. For the first procedure (12 patients), the solution instilled within the peritoneal cavity contained oxaliplatin, and a delay of 8–10 min was necessary to reach a temperature of 42–43°C. For the second procedure (12 patients), the cavity was initially filled only with the dextrose solution, and oxaliplatin was added to the peritoneal instillate when temperature reached 42–43°C. Plasma and peritoneal fluid oxaliplatin concentrations were analyzed according to a population pharmacokinetic approach using NONMEM.
Results
Peritoneal and total plasma data were simultaneously analyzed according to a three-compartment pharmacokinetic model. The peritoneal half-life ranged between 18 and 42 min. The mean peritoneal clearance was 5.47 L/h (±21%), and the mean plasma clearance was 3.71 L/h (±47%). The heated intra-operative procedure did not have any impact on oxaliplatin pharmacokinetics.
Conclusion
The inter-individual variability was larger for plasma pharmacokinetic parameters than that for peritoneal parameters. However, the percentage of oxaliplatin dose absorbed during a 30-min hyperthermic intraperitoneal chemotherapy may vary from 40 to 68%. The present pharmacokinetic model will be useful to implement pharmacokinetic evaluation of further clinical trials of hyperthermic intraperitoneal chemotherapy based on platinum compounds’ administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past two decades, the evolution of loco regional therapy has changed the management of peritoneal surface malignancies. Encouraging results of a randomized controlled study of hyperthermic intraperitoneal chemotherapy (HIPEC) in the management of peritoneal carcinomatosis in colorectal cancer patients have been published [10]. HIPEC has also been proposed in ovarian cancers, peritoneal mesothelioma and pseudomyxoma peritonei [9]. HIPEC administered in only one session, after complete cytoreduction under direct surgical monitoring, might be more applicable in clinical practice than in sequential intraperitoneal chemotherapy using permanent catheters [7].
Pharmacokinetics (PK) of heated intraperitoneal oxaliplatin have been previously reported [2–4]. However, no model has been used to analyze the data. The aim of the current study was to develop a PK model which is able to simultaneously describe peritoneal and plasma oxaliplatin concentrations versus time in order to obtain mean PK parameters and their interindividual variability. This model has been applied to compare the impact of two procedures of HIPEC on oxaliplatin PK.
Patients and methods
Patients and oxaliplatin administration
Data were obtained from 24 patients who were treated from June 2005 to January 2007 for primary or recurrent peritoneal carcinomatosis with debulking surgery and HIPEC. Their primitive tumor type was ovarian (n = 17), colorectal (n = 5), mesothelioma (n = 1), or pseudomyxoma (n = 1). All except the two patients with mesothelioma, or pseudomyxoma, were previously treated by intravenous chemotherapy.
All patients provided written informed consent before study enrolment. The main patient characteristics are shown in Table 1. HIPEC procedures were conducted as previously described by Elias et al. [4] with the “coliseum” technique. Oxaliplatin was administered in a 5% dextrose solution (2 L/m2) at a dose of 460 (n = 17) or 360 mg/m2 (n = 7). The initial dose (i.e., 460 mg/m2) was reduced for the final seven patients treated due to toxicity. The first 12 patients were treated according to the first HIPEC procedure (group 1), and the following 12 patients to the second procedure (group 2). For the first procedure, the solution instilled within the peritoneal cavity contained oxaliplatin, and a delay of 8–10 min was necessary to reach a temperature of 42–43°C. For the second procedure, the cavity was initially filled only with the dextrose solution, and oxaliplatin was added to the peritoneal instillate when the temperature reached 42–43°C. The extracorporeal circulation of the fluid was realized using the Performer LRT® System (Medolla, MO, Italy). Perfusion duration was exactly 30 min from the time when optimum temperature (42–43°C) was reached.
Sampling and platinum assay
Seven peritoneal fluid samples were collected (every 5 min during the 30-min HIPEC) per patient. An additional peritoneal sample was obtained just before the warm-up period for the first 12 patients (HIPEC procedure 1). Eleven blood samples (5 mL) were collected: before the HIPEC, 0.167, 0.333, 0.5, 0.75, 1, 2, 4, 6, and 8 after the beginning of the HIPEC. Blood samples were immediately centrifuged at 2,000×g for 10 min at 4°C. One milliliter of plasma was ultrafiltered using an Amicon MPS1 micro partition system with YMT membranes (30,000 MW cut-off) at 4°C for 20 min at 2,000×g.
Peritoneal fluid samples were collected: at the beginning of the perfusion (for the 12 first patients only), at the beginning of HIPEC, and every 5 minutes during HIPEC.
Samples were kept at −20°C until analysis by flameless atomic absorption spectrophotometry, according to a previously described method [8]. The limit of quantification was 10 ng/mL for both peritoneal fluid and ultrafiltrate, and 20 ng/mL for plasma.
Pharmacokinetic analysis
Peritoneal and total plasma data
Peritoneal and plasma data from the 24 patients were simultaneously analyzed using NONMEM (version V, level 1.1 running on Pentium 200 pro) [1] according to a three-compartment pharmacokinetic model (Fig. 1) and a first-order conditional estimation (FOCE) method. A proportional error model was used for residual and inter-patient variability. Individual pharmacokinetic parameters were obtained by Bayesian estimation using the POSTHOC option. The impact of the HIPEC technique (TECH = 0, or = 1 for the first or the second procedure, respectively) on absorption rate from peritoneal compartment to plasma, Ka, was tested according to the following equation: TVKa = θ5 × (1 − θ7 × TECH) where θ5 is the typical value of Ka (TVKa) of patients for whom TECH = 0, and θ5 × (1 − θ7) that of patients for whom TECH = 1. A first analysis was done by allocating a constant value of TECH (either 0 or 1) for all samples during the HIPEC procedure. A second analysis was done by coding TECH = 1 only for samples during the warm-up period and then TECH = 0. The two models with the covariate TECH were compared to that without covariate by the χ2 test of difference between the respective objective function values (OBJs); OBJ equal to minus twice the log likelihood of the data. A change of at least 3.84 (P < 0.05, one degree of freedom) was required to consider Ka as significantly dependent of the covariate TECH.
Ultrafiltrate plasma data
Since ultrafiltrate (uf) plasma concentrations observed at time 6 and/or 8 h were higher than those observed at previous sampling time for 12/24 patients, no attempt was made to obtain a structural pharmacokinetic model to describe the observed uf plasma data. The pharmacokinetic parameters corresponding to uf plasma concentrations were obtained by model independent method using Kinetica software (version 4.1, InnaPhase, Waltham, MA, USA). Mixed linear and logarithmic trapezoidal rule was used to calculate the area under the curve of the uf plasma concentrations versus time from time 0 to 8 h after the beginning of the HIPEC (AUC0-8 h).
Results
Pharmacokinetics
Peritoneal and total plasma data
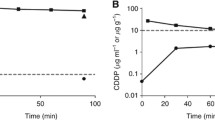
The structural pharmacokinetic model was found to describe the data very accurately with a residual variability of 8 and 13% for peritoneal and plasma concentrations, respectively. Figure 2 shows the mean observed peritoneal (Fig. 2a) and total plasma (Fig. 2b) concentrations versus time, and concentrations corresponding to mean pharmacokinetic parameters obtained by the model. The mean pharmacokinetic parameters and their interindividual variability are shown in Table 2. The inter-individual variability was larger for plasma pharmacokinetic parameters than that for peritoneal parameters. Figure 3 shows that the absorption rate (Ka) of oxaliplatin from peritoneal compartment to plasma was not dependent on the administered dose. The mean peritoneal half-life (=ln 2/Ka) ranged between 18 and 42 min.
By considering the covariate TECH during either the whole HIPEC procedure or only during the warm-up period, no significant decrease of the OBJ was observed indicating that there was no change in Ka due to the HIPEC technique. Figure 3 confirms that individual values of Ka were not dependent on the procedure.
Ultrafiltrate plasma data
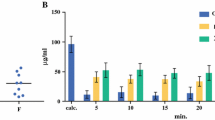
The mean uf plasma AUC0-8 h was 13.7 μg/mL × h (range: 8.0–20.0 μg/mL × h). As previously stated to justify the model-independent method to analyze these data, the mean uf concentration (Fig. 4) at T + 8 h was slightly higher than that at T + 6 h.
Toxicity
Major morbidity (grade 3 and 4) occurred in eight patients in group 1 (first HIPEC procedure), and in four patients in group 2 (second HIPEC procedure). The most significant morbidities were abdominal bleeding, anastomotic leaks, sensory alteration or paresthesia, infection complications, systemic inflammatory response syndrome, and thrombocytopenia. Eight patients in group 1 and one patient in group 2 developed abdominal bleeding. Grade 3–4 thrombocytopenia occurred in ten patients in group 1, and one patient in group 2. Systemic inflammatory response syndrome associated with clinical peritonitis and a high level of C reactive protein was seen in nine patients in group 1, and none in group 2.
Discussion
Pharmacokinetics of oxaliplatin given by HIPEC has been previously reported, but this study was the first based on a population approach allowing to analyze all peritoneal and plasma data from 24 patients simultaneously. The mean value of 29 min (range 18–42 min) obtained for the peritoneal half-life was consistent with those previously reported (i.e., 30 min [2] or 40 min [3]). The absorption process was not dependent on the dose (Fig. 3). The NONMEM methodology also allowed us to evaluate if the procedure had an impact on the absorption specifically during the warm-up period: Ka was not significantly different during this period in comparison with the period with peritoneal liquid at 42–43°C. Since the pharmacokinetic model well described the peritoneal data it is possible to estimate the mean absorbed dose of oxaliplatin according to the mean Ka and the duration of the HIPEC procedure: half of the dose is absorbed after 30 min of HIPEC. However, although the interindividual variability on Ka was limited (i.e., coefficient of variation of 22%), there was a ratio of 2 between the extreme values indicating that for some patients the percentage of dose absorbed during a 30-min HIPEC may vary between 40 and 68%. This difference contributed to the variability of the plasma oxaliplatin concentrations that was also the consequence of the interindividual variability in plasma pharmacokinetic parameters (i.e., 47% for plasma clearance).
The profile of ultrafiltered plasma oxaliplatin concentrations was unexpected. For most of the patients, concentrations at time 6 or 8 h were higher than those at previous sampling time. This was also observed in the experimental model of HIPEC in pigs [5]. We can make the hypothesis that platinum compounds are released from the peritoneal membranes lately after the end of the HIPEC. This fraction does not necessarily correspond to unchanged oxaliplatin. Indeed, we could make the hypothesis that oxaliplatin may be conjugated with glutathione by the glutathione-S-transferase within the cells constituting the peritoneal membranes and then transported out of the cells to the plasma compartment by the MRP transporters. Since platinum glutathione S-conjugates are relatively small molecules [6], they would contribute to this rebound of the uf plasma concentrations.
A significant correlation was observed between uf plasma AUC and platelet count at nadir (r = −0.58, P < 0.01, data not shown). However, the difference in hematologic toxicity may be explained by considering only the oxaliplatin dose: mean (±SD) nadir count of platelets was significantly lower (P < 0.001) after 460 mg/m2 of oxaliplatin than after 360 mg/m2: 86,900 (±69,600)/mm3 and 198,400 (±29,700)/mm3, respectively. The previous hypothesis relative to platinum glutathione S-conjugates could explain why pharmacokinetic–pharmacodynamic relationship is not stronger than that between dose and toxicity. Moreover, thrombopenia was also the consequence of post-operative hemorrhage that was more frequent for patients for whom the first HIPEC procedure was performed. Since all of them received 460 mg/m2 of oxaliplatin, whereas 7 of 12 patients with second HIPEC procedure received 360 mg/m2, HIPEC procedure probably contributed as much as the oxaliplatin doses or plasma concentrations to this hematological toxicity. Besides the pharmacokinetic results, this study showed the impact of the HIPEC procedure on toxicity. Detailed clinical (both toxicity and efficacy) results will be reported elsewhere.
In conclusion, the present pharmacokinetic model allowed us to quantify the respective interindividual variability to peritoneal and systemic processes. It will be useful to implement pharmacokinetic evaluation of further clinical trials of HIPEC based on oxaliplatin administration that is planned.
References
Beal SL, Sheiner LB (1982) Estimating population kinetics. Crit Rev Biomed Eng 8:195–222
Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, El OA, Pignon JP, Drouard-Troalen L, Ouellet JF, Ducreux M (2002) Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol 13:267–272
Elias D, Matsuhisa T, Sideris L, Liberale G, Drouard-Troalen L, Raynard B, Pocard M, Puizillou JM, Billard V, Bourget P, Ducreux M (2004) Heated intra-operative intraperitoneal oxaliplatin plus irinotecan after complete resection of peritoneal carcinomatosis: pharmacokinetics, tissue distribution and tolerance. Ann Oncol 15:1558–1565
Elias D, Raynard B, Bonnay M, Pocard M (2006) Heated intra-operative intraperitoneal oxaliplatin alone and in combination with intraperitoneal irinotecan: pharmacologic studies. Eur J Surg Oncol 32:607–613
Gesson-Paute A, Ferron G, Thomas F, de Lara EC, Chatelut E, Querleu D (2007) Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted Heated Intraoperative Intraperitoneal Chemotherapy (HIPEC): an experimental study. Ann Surg Oncol (available online)
Graham MA, Lockwood GF, Greenslade D, Brienza S, Bayssas M, Gamelin E (2000) Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res 6:1205–1218
Jaaback K, Johnson N (2006) Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev CD005340
LeRoy AF, Wehling ML, Sponseller HL, Friauf WS, Solomon RE, Dedrick RL, Litterst CL, Gram TE, Guarino AM, Becker DA (1977) Analysis of platinum in biological materials by flameless atomic absorption spectrophotometry. Biochem Med 18:184–191
Sugarbaker PH (2007) Adjuvant intraperitoneal chemotherapy: a review. Recent Results Cancer Res 169:83–89
Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA (2003) Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 21:3737–3743
Acknowledgment
We thank Miss Tracy Chapman for editorial assistance with the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferron, G., Dattez, S., Gladieff, L. et al. Pharmacokinetics of heated intraperitoneal oxaliplatin. Cancer Chemother Pharmacol 62, 679–683 (2008). https://doi.org/10.1007/s00280-007-0654-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0654-x