Abstract
Purpose: Cisplatin (CP)-induced nephrotoxicity is associated with the increased generation of reactive oxygen metabolites and lipid peroxidation in kidney, caused by the decreased levels of antioxidants and antioxidant enzymes. The purpose of this study was to evaluate the role of Spirulina, blue–green alga with antioxidant properties, in the protection of cisplatin-induced nephrotoxicity in rat. Methods: Rats were treated with CP (6 mg/kg bw, single dose, intraperitoneally). Spirulina (1,000 mg/kg) was administered orally for 8 days and CP treatment was given on day 4. Nephrotoxicity was assessed, 6 days after the CP treatment, by measuring plasma urea, creatinine, urinary N-acetyl-(d-glucose-aminidase) (β-NAG) and histopathology of kidney. Results: Rats treated with CP showed marked nephrotoxicity as evidenced from the significant elevation in plasma urea, creatinine and urinary β-NAG. Histological assessment revealed marked proximal tubular necrosis and extensive epithelial vacuolization in the kidney of CP-treated rats. Superoxide dismutase, catalase and glutathione peroxidase were decreased and lipid peroxidation was increased in kidney tissue. Pretreatment with Spirulina protected the rats from CP-induced nephrotoxicity. The rise in plasma urea, creatinine, urinary β-NAG, plasma and kidney tissue MDA and histomorphological changes were significantly attenuated by Spirulina. In vitro studies using human ovarian cancer cells revealed that Spirulina did not interfere with the cytotoxic effects of CP on tumor cells. Conclusions: In summary, Spirulina significantly protected the CP-induced nephrotoxicity through its antioxidant properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cisplatin (CP) is a potent antineoplastic drug, whose optimal clinical use is limited because of its ability to induce nephrotoxicity [1]. Acute renal failure has been observed after a single dose of cisplatin [2]. Primary targets of CP in kidney are proximal straight and distal convoluted tubules, where it accumulates and promotes cellular damage, and by involving multiple mechanisms including oxidative stress, DNA damage, apoptosis and inflammation [3–6]. Many in vivo and in vitro studies indicate an important role of reactive oxygen species (ROS) in the pathogenesis of nephrotoxicity [7]. CP induces free radical production causing oxidative renal damage, possibly due to depletion of non-enzymatic and enzymatic antioxidant systems [8]. Antioxidants have been shown to be protective in CP nephrotoxicity [9, 10]. Several radical scavengers and antioxidants such as vitamin E, vitamin C [11], selenium [12], edaravole [13], amifostine [14], superoxide dismutase (SOD) [10] and caffeic acid phenylethyl ester [15] are reported to attenuate CP-induced renal toxicity.
Spirulina (SP), a blue–green alga, is popularly used as a nutritional supplement as well as in therapeutic applications [16]. Spirulina contains proteins, lipids, carbohydrates, some vital minerals, vitamins including β-carotene and a pigmented protein, C-phycocyanin [17]. The antioxidant potential of Spirulina was demonstrated in both in vitro and in vivo studies [18]. Spirulina is known for its wide-ranging biological activities and antioxidative [18], anti-inflammatory [19], antimutagenic [20], antiviral [21], immune enhancing [18], cardioprotective [22] and anticancer properties [23].
Many studies have suggested that the plasma and tissue concentrations of various antioxidants including β-carotene were decreased during CP-based therapy in cancer patients and in experimental animal models [24], which, in part, may contribute to the development of nephroxicity by CP. Hence, the present study was carried out to determine the nephroprotective role of Spirulina against CP-induced nephrotoxicity in rats. Results revealed that Spirulina significantly attenuated the CP-induced nephrotoxicity in rats and further supported the crucial role of antioxidants in protecting nephrotoxicity induced by CP.
Materials and methods
Spirulina, a fine dark blue–green spray-dried powder, was prepared from Spirulina platensis (New Ambadi Estates, India). The biochemical analysis of Spirulina used in our experiments revealed a composition of proteins (65.38%), crude phycocyanin (15.37%), minerals (7.95%), total carotenoids (0.43%), β-carotene (0.17%) and total pheophorbide (0.02%). Required quantity of Spirulina powder was dissolved in sterile distilled water and used as the Spirulina preparation to treat the animals. Cisplatin was purchased from Sigma Chemical Co. (St. Louis, USA).
Animals and treatment
Adult male Wistar rats (weight 200–250 g) were used in the study. The rats were housed under conditions of controlled temperature and a 12-h lighting cycle and were fed with standard rat chow. The animals were divided into four groups of six animals each. The control group received only normal saline orally. The second group received Spirulina (1,000 mg/kg bw/day) orally for 8 days. The third group received a single dose of cisplatin (6 mg/kg bw) intraperitoneally and the fourth group received Spirulina (1,000 mg/kg bw/day) daily for 8 days and cisplatin (6 mg/kg bw, single dose, intraperitoneally) on day 4. On the fifth day after CP treatment, all the animals were kept in metabolic cages for 24 h urine collection. Blood was collected on the sixth day after CP treatment, by ocular puncture, for biochemical analysis and the kidneys were collected in buffered formalin for histopathological examination. The study was approved by the Institutional Ethics Committee for the use of laboratory animals at Nizam’s Institute of Medical Sciences.
Biochemical assays
Plasma urea and creatinine levels were measured spectrophotometrically by using commercially available kits. The urinary β-NAG activity was measured as per the reported procedure [25].
Lipid peroxidation products
Kidney tissue was homogenized (10%, w/v) in PBS (pH 7.4) containing 20-mmol/l butylated hydoxytoluene. The homogenate was centrifuged at 3,000 rpm for 10 min and the supernatant was mixed with equal volumes of 20% trichloroacetic acid, vortexed vigorously and centrifuged at 5,000 rpm for 30 min. To the protein-free supernatant, 0.33% thiobarbituric acid (TBA) was added and boiled for 1 h at 95°C. The TBA-reactive products were extracted in butanol and the intensity of the pink color was read at 520 nm [26]. Freshly diluted tetramethoxy propane (Sigma) was used as the standard and data were expressed in nmol of MDA/g of heart tissue.
Estimation of antioxidant enzymes
Superoxide dismutase activity in kidney tissue homogenate was determined spectrophotometrically according to the method of McCord and Fridovich [27]. This method is based on the ability of SOD to inhibit the reduction of cytochrome c in the presence of xanthine and xanthine oxidase. One unit was defined as the amount of enzyme that inhibits the reduction of cytochrome c by 50% and activity was expressed in units/mg protein. Catalase activity was determined by the method of Aebi [28], with H2O2 (10 mM) and phosphate buffer (0.05 M, pH 7.0) at 210 nm. A unit is defined as the amount of enzyme that catalyzed the dismutation of 1 μmol of H2O2/min. The specific activity is expressed in units/mg protein [28]. Glutathione peroxidase activity was measured by the NADPH oxidation method [29], and expressed as nmol of NADPH oxidized to NADP/mg protein. Protein was determined by method of Lowry et al. [30].
Histopathological studies
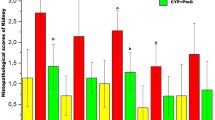
Histological sections (5 μm) of kidney from all the treated groups were stained with hematoxylin and eosin and periodic acid Schiff’s stain. The microscopic scoring of the kidney sections was carried out in a blinded fashion by a pathologist who is unaware of the treatment groups and assigned a score as described [31], which represents the approximate extent of necrotic area in the cortical proximal tubules on a scale of 0–4 (0, no necrosis; 1, a few focal necrotic spots; 2, necrotic area was about one half; 3, necrotic spots was about two thirds; 4, nearly the entire area was necrotic).
Effect of Spirulina on antitumor activity of CP in vitro
Human ovarian cancer cells were seeded into 96-well plates at a starting density of 2.5×104 cells/well and cultured overnight in phenol red-free RPMI-1640 medium, containing 5% fetal calf serum at 37°C, humidified with 5% CO2. The following day, CP (6 μM) and/or Spirulina (50 μg/ml) was added to the medium. Twenty-four and forty-eight hours later, 0.5% of MTT was added, incubated for 3 h, and then the medium was removed. The water-insoluble blue formazan dye formed was solubulized in DMSO, and the absorbance was read using a 96-well plate ELISA reader (Beckmann Coulter, AD 340) at 550 nm. All experiments were run in at least four parallels and repeated three times.
Statistical analysis
The statistical significance of differences among values of individual parameters was evaluated by using the Student’s t test. All the values are expressed as mean ± SD. The significance was set at P<0.05.
Results
To assess CP-induced nephrotoxicity, the plasma urea and creatinine levels were determined. Figure 1 shows significant elevation of plasma urea and creatinine levels in rats treated with CP alone as compared with untreated control (P<0.05). Urinary β-NAG, a marker of renal tubular damage, was also significantly increased in CP-treated animals (Fig. 1). In a dose–response study, we found that Spirulina at 1,000 mg/kg was the most effective dose in ameliorating the CP-induced nephrotoxicity (data not shown). Hence all our experiments were performed using this dose. The CP-induced rise in the levels of plasma urea, creatinine and urinary β-NAG was reduced significantly by Spirulina treatment. As shown in Figs. 2 and 3, there was a significant increase in lipid peroxidation in plasma and kidney tissues and a significant decrease in antioxidant enzymes, SOD, catalase and glutathione peroxidase in CP-treated animals. These findings are similar to those obtained by others, showing increased lipid peroxidation and decreased antioxidant enzymes in kidney tissues of CP-treated rats [15, 32]. However, pretreatment of Spirulina significantly attenuated the plasma and kidney tissue MDA levels. Furthermore, SOD, catalase and glutathione peroxidase levels were also restored to control levels.
Effect of Spirulina on cisplatin (CP)-induced nephrotoxicity as measured by: a plasma urea; b plasma creatinine; and c urinary β-NAG. Nephrotoxicity in rat was induced by CP (6 mg/kg bw single dose, intraperitoneally) and Spirulina (1,000 mg/kg) was administered orally, 3 days prior to CP treatment and continued till the end of the experiment. Values are expressed as mean ± SD (n=6), *P<0.05 versus control; **P<0.05 versus CP. The results show that Spirulina treatment attenuated CP-induced nephrotoxicity
Effect of Spirulina on cisplatin (CP)-induced lipid peroxidation (MDA). Rats were treated with CP and Spirulina as described in Fig. 1. Values are expressed as mean ± SD (n=6), *P<0.05 versus control; **P<0.05 versus CP. The results show that Spirulina treatment attenuated the CP-induced increase in lipid peroxidation
Effect of Spirulina on Cisplatin (CP)-induced changes in SOD, catalase and glutathione peroxidase in kidney tissue. Rats were treated with CP and Spirulina as described in Fig. 1. Values are expressed as mean ± SD (n=6), *P<0.05 versus control; **P<0.05 versus CP. The results show that Spirulina treatment attenuated the CP-induced decrease in SOD, catalase and glutathione peroxidase
The impaired renal function induced by CP was further confirmed by histological examination of kidney, from the regions of cortex and cortico-medullary junction (Fig. 4). As shown in Fig. 4a, kidney from control rats showed no abnormality whereas kidney in CP-treated animals revealed a marked proximal tubular necrosis, extensive epithelial vacuolization, swelling and tubular dilation and renal tubules with hyaline casts (Fig. 4b). The glomerulus and tubular epithelial changes were less severe in the group treated with CP + Spirulina when compared with CP-treated rats (Fig. 4c). The histological changes were graded as described in the Materials and methods section and are summarized in Table 1.
Histological examination of rat kidney. Left panel shows cortical region (H&E 40×); Right panel shows cortico-medullary junction (H&E, 100×). a Control rat. Left and right panels show normal morphology; b cisplatin-treated rat. Left and right panels show renal tubules with hyaline casts, swelling and vacuolization and proximal tubular necrosis; and c cisplatin + Spirulina-treated rat. Left and right panels show minimal tubular necrosis
Though the urea and creatinine levels were not normal in Spirulina + CP-treated rats, they were significantly less as compared with CP-treated rats. However, histopathological data and urinary β-NAG (indicative of tubular necrosis) clearly indicated that Spirulina protected against CP-induced nephrotoxicity.
To evaluate whether Spirulina could modify the chemotherapeutic efficacy of CP, we studied the effect of Spirulina on CP-induced cell-killing in human ovarian cancer cells in vitro. The results demonstrated that the cell survival was reduced significantly with CP treatment after 24 and 48 h of incubation, and co-administration of CP with Spirulina had no significant effect on CP-induced cell death (Fig. 5).
Effect of Spirulina (SP) on the antitumor potency of CP. Human ovarian cancer cells were plated in 96-well plates and allowed to attach overnight. The cells were treated with CP and/or Spirulina for a period of 24 or 48 h and cell proliferation was determined by MTT assay. Control cell growth was set at 100%. Values are expressed as mean ± SD (n=3), *P<0.01 versus control; **P<0.001 versus control. The results show that Spirulina does not interfere with the cytotoxic effect of CP on tumor cells
Discussion
The production of ROS and oxidative stress in kidney tissue have been implicated in the pathogenesis of CP-induced renal injury [33] including lipid peroxidation, enzyme inactivation, changes in the cellular non-enzymatic and enzymatic antioxidant system [10, 15, 31]. It has been reported that the administration of CP to rats resulted in the depletion of GSH in kidney tissue [34]. CP has been shown to inhibit the function of mitochondrial respiratory complex in normal tubular cells [35], resulting in ROS generation and mitochondrial dysfunction [36]. The involvement of oxidative stress was further supported by the fact that antioxidants such as melatonin [37], vitamin E, vitamin C [11], selenium [12], diethyldithiocarbamate [32] and MnSOD prevented CP-induced nephrotoxicity [10]. Overexpression of heme oxygenase-1 (HO-1) ameliorated [38] the renal damage induced by CP, while deficiency of HO-1 [39] worsened it, supporting the involvement of oxidative stress in the experimental model of CP toxicity. The protective effect of dimethylthiourea (·OH radical scavenger) against CP-induced proximal tubular (LLC-PK1) cell toxicity in vitro, also demonstrated the crucial role of ROS in CP-induced toxicity [7].
Pretreatment of Spirulina attenuated CP-induced nephrotoxicity and this effect is attributed to its antioxidant property. Several studies have demonstrated that Spirulina possess significant antioxidant activity both in vitro and in vivo [22, 40–42]. Earlier studies have shown that Spirulina exhibits antioxidant property in various oxidative conditions that can cause tissue injury [18, 43–45]. Spirulina prevented the cyclophosphamide and mitomycin-C toxicity [45] and CP- and urethane-induced genotoxicity in mice [46]. Rats fed with a Spirulina-enriched diet showed reduced ischemia–reperfusion-induced cerebral infarction [47]. In our recent studies, we have demonstrated that mice pretreated with Spirulina significantly protected doxorubicin-induced cardiotoxicity [22], and also attenuated doxorubicin-induced ROS generation and apoptosis in isolated rat cardiomyocytes in vitro [42]. Recent study has demonstrated that C-phycocyanin, one of the main constituents of Spirulina, inhibited oxalate-mediated lipid peroxidation and protected renal cell injury [48]. In addition, the results of the present study also indicated that Spirulina did not interfere with the inhibition of cell proliferation induced by CP in human ovarian cancer cells in vitro.
Though the results of the present study suggest Spirulina offers protection against CP-induced nephrotoxicity through the inhibition of oxidative stress, other possible mechanism(s) cannot be ruled out. It is evident from many studies that apoptosis is involved in CP-induced renal injury [6, 49]. A recent study has indicated that treatment with a Spirulina-enriched diet reduced the ischemia–reperfusion-induced apoptosis and cerebral infarction by inhibiting caspase-3 activity [47]. The chemoprotective agent, N-acetylcysteine blocks the CP-induced apoptosis through the caspase signaling pathway [50]. In our recent study, we have demonstrated that Spirulina and C-phycocyanin significantly inhibited the doxorubicin-induced free radical generation and apoptosis by attenuating caspase-3 activity in isolated rat cardiomyocytes [42]. It is likely that Spirulina might be altering the oxidative stress-mediated apoptotic pathway, thereby inhibiting the CP-induced apoptosis. However, this requires further investigation.
In summary, our study demonstrated that Spirulina protected CP-induced nephrotoxicity in rats by its antioxidant properties. Furthermore, Spirulina does not compromise the antitumor effect of CP. Further studies are required to examine the clinical use and exact mechanisms behind a possible protective effect of Spirulina on CP-induced nephrotoxicity.
References
Loehrer PJ, Einhorn LH (1984) Drugs five years later Cisplatin. Ann Intern Med 100:704–713
Heidemann HT, Muller S, Mertins L, Stepan G, Hoffmann K, Ohnhaus EE (1989) Effect of aminophylline on cisplatin nephrotoxicity in the rat. Br J Pharmacol 97:313–318
Safirstein R, Winston J, Moel D, Dikman S, Guttenplan J (1987) Cisplatin nephrotoxicity: insights into mechanism. Int J Androl 10:325–346
Schaaf GJ, Maas RF, de Groene EM, Fink-Gremmels J (2002) Management of oxidative stress by heme oxygenase-1 in cisplatin-induced toxicity in renal tubular cells. Free Radic Res 36:835–843
Xiao T, Choudhary S, Zhang W, Ansari NH, Salahudeen A (2003) Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells. J Toxicol Environ Health A 66:469–479
Cummings BS, Schnellmann RG (2002) Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302:8–17
Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV (1998) In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int 53:394–401
Rybak LP, Ravi R, Somani SM (1995) Mechanism of protection by diethyldithiocarbamate against cisplatin ototoxicity: antioxidant system. Fundam Appl Toxicol 26:293–300
Antunes LM, Araujo MC, Darin JD, Bianchi ML (2000) Effects of the antioxidants curcumin and vitamin C on cisplatin-induced clastogenesis in Wistar rat bone marrow cells. Mutat Res 465:131–137
Davis CA, Nick HS, Agarwal A (2001) Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol 12:2683–2690
Appenroth D, Frob S, Kersten L, Splinter FK, Winnefeld K (1997) Protective effects of vitamin E and C on cisplatin nephrotoxicity in developing rats. Arch Toxicol 71:677–683
Antunes LM, Francescato HD, Darin JD, de Lourdes PBM (2000) Effects of selenium pretreatment on cisplatin-induced chromosome aberrations in wistar rats. Teratog Carcinog Mutagen 20:341–348
Sueishi K, Mishima K, Makino K, Itoh Y, Tsuruya K, Hirakata H, Oishi R (2002) Protection by a radical scavenger edaravone against cisplatin-induced nephrotoxicity in rats. Eur J Pharmacol 451:203–208
Orditura M, De Vita F, Roscigno A, Infusino S, Auriemma A, Iodice P, Ciaramella F, Abbate G, Catalano G (1999) Amifostine: a selective cytoprotective agent of normal tissues from chemo-radiotherapy induced toxicity (Review). Oncol Rep 6:1357–1362
Ozen S, Akyol O, Iraz M, Sogut S, Ozugurlu F, Ozyurt H, Odaci E, Yildirim Z (2004) Role of caffeic acid phenethyl ester, an active component of propolis, against cisplatin-induced nephrotoxicity in rats. J Appl Toxicol 24:27–35
Khan Z, Bhadouria P, Bisen PS (2005) Nutritional and therapeutic potential of Spirulina. Curr Pharm Biotechnol 6:373–379
Ciferri O (1983) Spirulina, the edible microorganism. Microbiol Rev 47:551–578
Miranda MS, Cintra RG, Barros SB, Mancini Filho J (1998) Antioxidant activity of the microalga Spirulina maxima. Braz J Med Biol Res 31:1075–1079
Remirez D, Gonzalez R, Merino N, Rodriguez S, Ancheta O (2002) Inhibitory effects of Spirulina in zymosan-induced arthritis in mice. Mediators Inflamm 11:75–79
Chamorro G, Salazar M, Favila L, Bourges H (1996) Pharmacology and toxicology of Spirulina alga. Rev Invest Clin 48:389–399
Ayehunie S, Belay A, Baba TW, Ruprecht RM (1998) Inhibition of HIV-1 replication by an aqueous extract of Spirulina platensis (Arthrospira platensis). J Acquir Immune Defic Syndr Hum Retrovirol 18:7–12
Khan M, Shobha JC, Mohan IK, Naidu MU, Sundaram C, Singh S, Kuppusamy P, Kutala VK (2005) Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother Res 19:1030–1037
Schwartz J, Shklar G, Reid S, Trickler D (1988) Prevention of experimental oral cancer by extracts of Spirulina–Dunaliella algae. Nutr Cancer 11:127–134
Weijl NI, Hopman GD, Wipkink-Bakker A, Lentjes EG, Berger HM, Cleton FJ, Osanto S (1998) Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Ann Oncol 9:1331–1337
Horak E, Hopfer SM, Sunderman FW Jr (1981) Spectrophotometric assay for urinary N-acetyl-beta-d-glucosaminidase activity. Clin Chem 27:1180–1185
Bernheim FM, Bernheim BL, Wilbur KM (1948) The reaction between TBA and the oxidation products of certain lipids. J Biol Chem 174:257–264
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Baliga R, Ueda N, Walker PD, Shah SV (1999) Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev 31:971–997
Somani SM, Ravi R, Rybak LP (1995) Diethyldithiocarbamate protection against cisplatin nephrotoxicity: antioxidant system. Drug Chem Toxicol 18:151–170
Yilmaz HR, Uz E, Yucel N, Altuntas I, Ozcelik N (2004) Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J Biochem Mol Toxicol 18:234–238
Nakano S, Gemba M (1989) Potentiation of cisplatin-induced lipid peroxidation in kidney cortical slices by glutathione depletion. Jpn J Pharmacol 50:87–92
Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF (1997) Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther 280:638–649
Richter C, Gogvadze V, Laffranchi R, Schlapbach R, Schweizer M, Suter M, Walter P, Yaffee M (1995) Oxidants in mitochondria: from physiology to Diseases. Biochim Biophys Acta 1271:67–74
Sener G, Satiroglu H, Kabasakal L, Arbak S, Oner S, Ercan F, Keyer-Uysa M (2000) The protective effect of melatonin on cisplatin nephrotoxicity. Fundam Clin Pharmacol 14:553–560
Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A (2000) Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol 278:F726–F736
Agarwal A, Nick HS (2000) Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol 11:965–973
Bhat VB, Madyastha KM (2000) C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro. Biochem Biophys Res Commun 275:20–25
Romay C, Armesto J, Remirez D, Gonzalez R, Ledon N, Garcia I (1998) Antioxidant and anti-inflammatory properties of C-phycocyanin from blue–green Algae. Inflamm Res 47:36–41
Khan M, Varadharaj S, Ganesan LP, Shobha JC, Naidu MU, Parinandi NL, Tridandapani S, Kutala VK, Kuppusamy P (2006) C-phycocyanin protects against ischemia–reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. Am J Physiol Heart Circ Physiol (in press)
Mathew B, Sankaranarayanan R, Nair PP, Varghese C, Somanathan T, Amma BP, Amma NS, Nair MK (1995) Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutr Cancer 24:197–202
Premkumar K, Pachiappan A, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A (2001) Effect of Spirulina fusiformis on cyclophosphamide and mitomycin-C induced genotoxicity and oxidative stress in mice. Fitoterapia 72:906–911
Upasani CD, Khera A, Balaraman R (2001) Effect of lead with vitamin E, C, or Spirulina on malondialdehyde, conjugated dienes and hydroperoxides in rats. Indian J Exp Biol 39:70–74
Premkumar K, Abraham SK, Santhiya ST, Ramesh A (2004) Protective effect of Spirulina fusiformis on chemical-induced genotoxicity in mice. Fitoterapia 75:24–31
Wang Y, Chang CF, Chou J, Chen HL, Deng X, Harvey BK, Cadet JL, Bickford PC (2005) Dietary supplementation of with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp Neurol 193:75–84
Farooq SM, Asokan D, Sakthivel R, Kalaiselvi P, Varalakshmi P (2004) Salubrious effect of C-phycocyanin against oxalate-mediated renal cell injury. Clin Chim Acta 348:199–205
Lau AH (1999) Apoptosis induced by cisplatin nephrotoxic injury. Kidney Int 56:1295–1298
Wu YJ, Muldoon LL, Neuwelt EA (2005) The chemoprotective agent N-acetylcysteine blocks cisplatin-induced apoptosis through caspase signaling pathway. J Pharmacol Exp Ther 312:424–431
Acknowledgment
We thank M/s Parry Neutraceuticals, Chennai, India for providing the pure powder of Spirulina for our study and Jing Fang for her help in the study with ovarian cancer cells. This work was partially supported by the National Institutes of Health Grant CA-102264.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohan, I.K., Khan, M., Shobha, J.C. et al. Protection against cisplatin-induced nephrotoxicity by Spirulina in rats. Cancer Chemother Pharmacol 58, 802–808 (2006). https://doi.org/10.1007/s00280-006-0231-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0231-8