Abstract
Background: Tumour growth is dependent on angiogenesis. Antiangiogenic chemotherapy, i.e. continuous or metronomic low-dose chemotherapy, is a method for administrating cytostatics at a low and well-tolerated concentration without prolonged breaks. The target is the genetically stable endothelial cells playing a pivotal role in angiogenesis within the tumour. Different mediators could mediate the antiangiogenic effect of metronomic chemotherapy. One of these mediators could be thrombospondin (TSP). TSP is a potent inhibitor of angiogenesis and might therefore be important in controlling tumour growth. This study was designed to evaluate the effects of low-dose continuous or moderate-dose bolus chemotherapy on tumour growth and on tumour expression of TSP. Materials and methods: Rats bearing a malignant prostate tumour (Dunning AT-1) not expressing TSP were treated systemically with cyclophosphamide, doxorubicin or paclitaxel and the combination of cyclophosphamide and doxorubicin. Tumour growth and body weight were measured during the treatment. CD36, one of TSP’s main receptors, was also analysed. The expression pattern of TSP-1, TSP-2 and CD36 was investigated using immunohistochemistry and Western blot analyses. Q-PCR was used to analyse TSP-1 mRNA expression. Results: Low-dose cyclophosphamide and paclitaxel re-induced the expression of TSP in the tumours. However, following a bolus dose of doxorubicin, tumours showed no expression of TSP. Both cyclophosphamide and doxorubicin treatments decreased the tumour weight by more than 60% compared with vehicle controls. When cyclophosphamide and doxorubicin were combined the tumour weight was reduced by 47%, while paclitaxel reduced the tumour weight by 18% compared to the vehicle controls. Conclusions: Systemic low-dose continuous treatment of a rat prostate cancer model with cyclophosphamide and paclitaxel induced the expression of TSP in tumour tissue and inhibited tumour growth. These findings support the hypothesis that the anti-tumour effect of low-dose metronomic chemotherapy, at least with certain chemotherapeutics, is partly mediated by induction of endogenous antiangiogenic factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy has been the predominant medical cancer treatment, not counting surgery, for the last decades. Cytotoxics are designed to kill or inhibit the proliferation of rapidly dividing cancer cells. However, recent studies have shown that cytotoxics may have anti-tumour effect also by suppressing tumour angiogenesis [1].
Angiogenesis is the formation of new micro-vessels from existing ones and these newly formed vessels contain dividing endothelial cells [2]. Therefore, the endothelial cells would be expected to be sensitive to chemotherapy, particularly as they are directly exposed to the agent used. Conventionally, chemotherapeutics are administrated in a single dose or short courses of therapy at the highest doses possible without causing life-threatening toxicity, so-called maximum tolerated dose (MTD). During the prolonged breaks between the treatment periods, the transient effect on endothelial cells may be reversed by different mechanisms. Consequently, Browder et al. [3] suggested a strategy to optimize the antiangiogenic effects of chemotherapy by continuous administration of the drug at considerably lower doses than the MTD [4].
The antiangiogenic effect of continuous or metronomic chemotherapy could be mediated by different mediators. Recently, Bocci et al. [5] showed that TSP-1 may be one of the mediators involved. TSP-1 is a 450 kDa glycoprotein belonging to a family of five members. TSP is a multifunctional protein and activated by tumour suppressor gene products such as p53 [6]. TSP-1 is expressed by different cells, including perivascular cells, stroma cells and platelets [7], and can under defined conditions inhibit endothelial cell growth. In addition to these activities, the intact TSP-1 molecule, as well as specific peptide fragments, is able to induce apoptosis in endothelial cells. In humans, TSP is down-regulated during prostate cancer development [8–10] and disappears in cancer tissues. This result was confirmed in a rat model with different sub-lines of various prostate tumour grades [11]. It has also been shown in previous experiments using the same experimental system as in the present study that continuous/metronomic treatment with paclitaxel exerts antiangiogenic effects per se in tumour-free tissues as well as antiangiogenic and anti-tumour effects on the AT-1 rat prostate cancer model [12]. In these experiments, paclitaxel was administered as a subcutaneous (s.c.) continuous infusion, which may be considered as an extreme variant of metronomic scheduling. In the present study, the effects on growth and TSP expression were studied in the syngeneic AT-1 Dunning prostate cancer following systemic low-dose continuous or moderate-dose bolus chemotherapy.
Materials and methods
Tumour tissue implantation and preparation
The syngeneic rat Dunning sub-lines represent different prostate tumour grades [13]. In the present study we have used AT-1, a fast growing, androgen-insensitive, anaplastic adenocarcinoma with low and moderate metastatic capacity. AT-1 cells were grown in culture as a monolayer for 2 weeks before implanted (2×106 cells) subcutaneously into adult male Copenhagen rats. The rats were housed in a controlled environment and fed ad libitum. At the end of the experiment, the animals were killed and all tumour tissues were quickly removed and weighed. Part of the tumour was frozen in liquid nitrogen and stored in −80°C prior to protein and RNA extraction or fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight before imbedding in paraffin.
Drug treatment
The animals were divided into five groups containing 12–16 animals in each group. All continuous chemotherapies were given using subcutaneous osmotic pumps (model no. 2ML1; Alzet Osmotic pumps, Mountain View, CA, USA) as described previously [11]. Controls were treated with the vehicle (0.9% w/v NaCl, saline). Groups were treated with either (1) 100 mg/kg/week of cyclophosphamide for 10 days, (2) 4 mg/kg of doxorubicin as an intravenous (i.v.) injection bolus dose (controls received i.v. injection of the vehicle), (3) 19 mg/kg/week of paclitaxel for 10 days or (4) combination of 100 mg/kg/week of cyclophosphamide s.c. and 4 mg/kg/week doxorubicin as an i.v. injection bolus dose. The doses used were chosen based on our previous experience in two rat strains, the Sprague–Dawley and the Copenhagen rats, in which we aimed at finding doses that only marginally affected physiological body weight gain, which adult rats display [12, 14]. It is noteworthy that adult rats grow physiologically considerably faster than adult mice. As a result, the general toxic effects of chemotherapy on body weight gain should be a much more sensitive indicator in rats than in mice. All treatments started on day 4 after tumour cell implantation and continued for 10 days. The size of the tumour and the weight of the animals were measured every third day, as described previously [11].
Immunohistochemistry
De-paraffinated sections of the tumour were heated in an antigen unmasking solution (Vector Laboratories, Inc., CA, USA) followed by incubation in normal serum for 30 min and thereafter for 1 h with the primary antibodies. The primary antibodies used were mouse monoclonal anti-thrombospondin-1 (Cat # MS-421, Clone A6.1, Neomarker, CA, USA), goat polyclonal anti-thrombospondin-2 and goat polyclonal anti-CD36 (Cat # sc-12313, Cat # sc-5522, Santa Cruz Biotechnology, Inc., USA). After incubation with a biotinylated secondary antibody, the sections were incubated with ABC reagents and peroxidase substrate for development. The sections were counterstained with Mayer’s haematoxylin solution.
A blocking peptide was used for competition studies (Cat # sc-12312 P, Santa Cruz Biotechnology, Inc.). The TSP-1 primary antibody was mixed and incubated for 2 h with the blocking peptide and then incubated for 1 h on the slides. Positive control slides for TSP-1 were from Neomarker (Cat # MS-421-pcs). As negative controls, sections from each tumour were processed in the same way as above but without the primary antibody whereas normal serum was added to the section. Three sections were stained for each sample.
Immunoblot analysis
The tissues were grounded with a homogenizer and suspended in lysis buffer containing complete protease inhibitors. The protein extracts were separated by electrophoreses in SDS-polyacrylamide gels and blotted onto a PVDF membrane by wet transfer. The membrane was analysed with antibodies against thrombospondin-1 (Cat # MS-421, Neomarker), thrombospondin-2 and CD36 (Cat # sc-12313, Cat # sc-5522, Santa Cruz Biotechnology, Inc). The blot was visualized by enhanced chemiluminescence technique, ECL (Amersham, Sweden). Positive control for TSP was purchased from Neomarker (Cat # MS-421-PCL) and for CD36 HUVEC cells were used.
RNA and cDNA isolation
The TRIzol extraction method (Life Technologies AB, Täby, Sweden) was used to isolate total RNA from frozen tissues of AT-1 tumours. Total RNA concentrations were determined by spectrophotometer counting. RNA concentration and integrity were ensured by ethidiumbromide staining after agarose gel electrophoresis.
First strand cDNA was synthesized using 2 μg RNA, random hexamers and M-MLV enzyme (Promega).
PCR and primers
Real-time quantitative reverse transcription (RT) PCR was performed using ABI PRISM 7000 (Applied Biosystems, Applera Corporation, USA). RT-PCR amplification mixture contained template cDNA, 2× syber Green master mix or 2× TaqMan master mix (Applied Biosystems) and forward and reverse primers.
RT-PCR parameters were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Chemicals were from Applied Biosystems (Applera Corporation). Primers and probe for 18s and TSP-1 were purchased from Applied Biosystems (# 4310893E and # Hs00170236, Applera Corporation). Primers for TSP-1 were synthesized by Cybergene AB (Huddinge, Sweden). The primers are from rat sequences. Relative mRNA quantification was performed using the ΔΔCT method normalized to 18S and expressed as a fold difference compared with untreated tumours.
-
TSP-1
-
Forward primer 5′ CACGCTACAGGACAGCAT 3′
-
Reverse primer 5′ GGCCGCCTCAGCTCATT 3′
-
Statistics
The non-parametric Mann–Whitney U test for unpaired (two-tailed) observations was used. For comparison of the tumour growth rate between treatment groups, the Kruskal–Wallis test was used. The criterion for statistical significance was P<0.05.
Ethical considerations
The local ethics committee for animal research approved the animal experiments. Care was taken not to expose animals to unnecessary suffering and the number of animals was kept low.
Results
Effect of cyclophosphamide, doxorubicin and paclitaxel on body weight
Cyclophosphamide treatment decreased body weight by 7% (P=0.01) compared with controls on the day of killing. After treatment with doxorubicin, body weight decreased by 11% (P=0.0002) and when cyclophosphamide and doxorubicin were combined the reduction of body weight was 21% (P=0.0001). Body weight decrease after paclitaxel treatment was 5% compared to the control group (P=0.006).
Effect of cyclophosphamide, doxorubicin and paclitaxel on tumour weight
Treatment of the animals with cyclophosphamide reduced the tumour weight by 64% (P<0.0005) compared to the control. Doxorubicin treatment decreased the tumour weight by 68% (P<0.0002) and when cyclophosphamide and doxorubicin were combined the tumour weight was reduced by 47% (P<0.0006). Tumour weight was not significantly decreased (18%, P=0.60) after paclitaxel treatment compared to the control (Fig. 1). However, paclitaxel treatment reduced the tumour growth rate significantly (P<0.05). Data from tumour growth and tumour growth rate after paclitaxel treatment in the same experimental setup have been published previously [12].
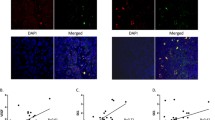
Effect on tumour weight after systemic treatment with cyclophosphamide, paclitaxel, doxorubicin or the combination of cyclophosphamide and doxorubicin. The values are given in percent of vehicle control; bars indicate SEM. The cyclophosphamide dose was 100 mg/kg/week, while the paclitaxel dose was 16 mg/kg/week administrated via an s.c. osmotic pump for 10 days. The doxorubicin dose was 4 mg/kg and given as a bolus dose. The treatment with cyclophosphamide, doxorubicin and the combination of cyclophosphamide and doxorubicin reduced the tumour weight significantly compared to the vehicle control (P<0.01). Paclitaxel did not exert any significant effect (P=0.60). Data on the effect on tumour growth after paclitaxel treatment in the same experimental system have been published previously [12]
Western blot analysis of thrombospondin
Western blot analysis showed an up-regulation of TSP-1 in the AT-1 tumours after treatment with cyclophosphamide and paclitaxel. However, there was no expression of TSP-1 in the AT-1 tumour in animals receiving doxorubicin as a bolus dose or in the control tumours (Fig. 2a, b). Analysis of protein expression of CD36 receptor and TSP-2 revealed no difference between treated animals and vehicle controls.
a Western blot analysis of TSP-1 in untreated and treated AT-1 bearing animals. Lane 1 TSP control, lanes 2–6 treatment with doxorubicin as a bolus dose, lanes 7 and 8 treatment with vehicle, lanes 9–13 treatment with cyclophosphamide with osmotic pump. It is a representative picture. b Western blot analysis of TSP-1 in untreated and treated AT-1 bearing animals. Lane 1 TSP control, lanes 2–8 treatment with vehicle, lane 9 TSP control, lanes 10–16 treatment with paclitaxel with osmotic pump. It is a representative picture
Quantitative real-time PCR analysis of thrombospondin
The protein analysis was supported by mRNA expression analysed by PCR. An increased expression of TSP-1 mRNA was seen after treatment with cyclophosphamide and paclitaxel compared to control. After bolus treatment with doxorubicin there was a minor increase of TSP-1 mRNA expression. In vehicle controls, no TSP-1 mRNA was found (Fig. 3).
Real-time quantitative RT-PCR analysis of TSP-1 mRNA expression in untreated tumour tissue or treated tumour tissue with cyclophosphamide (with osmotic pump), doxorubicin (as a bolus dose) or paclitaxel (with osmotic pump). Quantitative real-time RT-PCR was performed on total RNA. The gene values were normalized to the level of 18s in respective tissues. The expression of TSP-1 mRNA is given as relative values to control. Values are given as median and bars indicate the first and third quartiles with 5–6 animals in each group
Immunohistochemistry analysis
The expression of TSP-1 in AT-1 tumours treated with cyclophosphamide and paclitaxel was confirmed with immunohistochemistry. There was no expression of TSP in the tumour specimens prepared from vehicle controls (Fig. 4).
Analysis of thrombospondin-1 by immunohistochemistry in untreated and treated AT-1 bearing animals (×200 magnification). a Thrombospondin-1 positive AT-1 Dunning tumour tissue treatment with cyclophosphamide (with osmotic pump). b No detectable thrombospondin-1 expression in AT-1 Dunning tumour tissue treatment with doxorubicin (as a bolus dose). c Thrombospondin-1 positive AT-1 Dunning tumour tissue treatment with paclitaxel (with osmotic pump). d No detectable thrombospondin-1 expression in AT-1 Dunning tumour tissue treatment with vehicle alone
Discussion
This study demonstrated that continuous s.c. infusion, the extreme of metronomic treatment, of low-dose cyclophosphamide and paclitaxel chemotherapy significantly reduced tumour size and, interestingly, re-induced the expression of the antiangiogenic factor TSP in the tumours.
Conventional chemotherapy MTD scheduling, given cyclically with long interruptions, could offset any initial antiangiogenic effect of cytotoxics resulting in an insufficient overall anti-tumour effect. As antiangiogenic effects cause anti-tumour effects, low-dose chemotherapy given continuously could enhance the antiangiogenic effect resulting in a marked anti-tumour effect [3]. Conventional chemotherapy in the treatment of prostate cancer has shown a moderate palliative response with lowering of PSA levels but with no clear-cut prolongation of survival time [15].
We have previously reported antiangiogenic and anti-tumour effects of treatment with cytotoxic drugs in rats following metronomic or bolus treatment with paclitaxel, cyclophosphamide and doxorubicin [12, 14, 16]. Although the molecular mechanisms involved in the antiangiogenic and anti-tumour effects of continuous chemotherapy are still not fully known, the effects could to some degree be explained by the introduction of the angiogenetic inhibitor TSP-1 as shown here for rat prostate cancer, or additional antiangiogenic endogenous factors. Induction of TSP-1 after metronomic treatment with cyclophosphamide was recently demonstrated in Lewis lung carcinoma and B16 melanoma tumours in mice [7]. The cellular mechanism for this induction of TSP-1 by continuous low-dose chemotherapy still awaits elucidation. It is, however, well known that chemotherapeutic drugs trigger signal transduction pathways leading to the induction of mediators of cell-cycle arrest and apoptosis.
A micro-array analysis by Yoo et al. [17] showed an induced change in gene expression in docetaxel-treated head and neck squamous cell carcinoma. Interestingly, TSP was one of the genes affected. DNA damaging agents such as chemotherapeutics may induce/up-regulate the p53 suppressor gene, which in turn causes enhanced expression of TSP [18]. Moreover, activation of Ras signal pathway can decrease TSP-1 expression as described in a prostate tumour model [19]. Therefore, agents targeting pathways of this kind may induce an increase in TSP expression. The reasons for TSP re-induction in the present study after cyclophosphamide and paclitaxel treatment could thus be due to inhibition or activation of different pro- or antiangiogenic pathways. Another way by which low-dose chemotherapy could induce an antiangiogenic effect is by decreasing the viability of circulating bone marrow-derived endothelial precursor cells [20–22] and therefore act in a similar way as other antiangiogenic agents, including endostatin and angiostatin [23, 24].
In the present study, doxorubicin did not re-induce the expression of TSP. This could be due to the fact that this drug was given as a moderate-dose bolus and not as continuous low-dose treatment or, alternatively, because of a drug-specific feature. Clearly, induction of TSP is not the only mechanism for decreasing tumour weight, since a significant decrease in tumour weight occurred also following bolus treatment with doxorubicin despite the absence of TSP expression in the AT-1 tumour.
It is suggested that continuous/metronomic therapy may achieve a more pronounced anti-tumoural effect and significantly diminish the side effects compared with conventional bolus MTD-type chemotherapy. Importantly, as recently reported, the long-term overall anti-tumour success with metronomic chemotherapy in a stringent transgenic mouse model is markedly improved by using a so-called “chemo-switch” protocol. Such a protocol involves sequential chemotherapy administered as maximum-tolerated dose succeeded by metronomic chemotherapy and overlaid with multitarget inhibition of both platelet-derived growth factor, which disrupts pericyte support of tumour endothelial cells, and VEGF [25]. This emphasizes that exploration of the antiangiogenic and anti-tumour effects of metronomic treatment of various chemotherapeutics in different preclinical animal and tumour models is an important part in the continuous quest of developing regiments that can be used in the clinic. Clearly, more pre-clinical studies are needed in order to elucidate drug-specific features and to optimize the dose and treatment schedule in different animal models of different tumour types. Moreover, combinations with chemotherapeutics and various other types of drugs, such as Avastin®, an antibody against VEGF that inhibits angiogenesis, may enhance the anti-tumour effect and thus improve the survival time in cancer patients [26].
References
Miller KD, Sweeney CJ, Sledge GW Jr (2001) Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol 19(4):1195–1206
Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG (2000) Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res 60(5):1388–1393
Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS et al (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60(7):1878–1886
Bergers G, Hanahan D (2002) Combining antiangiogenic agents with metronomic chemotherapy enhances efficacy against late-stage pancreatic islet carcinomas in mice. Cold Spring Harb Symp Quant Biol 67:293–300
Bocci G, Francia G, Man S, Lawler J, Kerbel RS (2003) Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA 100(22):12917–12922
Lawler J (2000) The functions of thrombospondin-1 and -2. Curr Opin Cell Biol 12(5):634–640
Hamano Y, Sugimoto H, Soubasakos MA, Kieran M, Olsen BR, Lawler J et al (2004) Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res 64(5):1570–1574
Doll JA, Reiher FK, Crawford SE, Pins MR, Campbell SC, Bouck NP (2001) Thrombospondin-1, vascular endothelial growth factor and fibroblast growth factor-2 are key functional regulators of angiogenesis in the prostate. Prostate 49(4):293–305
Kwak C, Jin RJ, Lee C, Park MS, Lee SE (2002) Thrombospondin-1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int 89(3):303–309
Vallbo C, Wang W, Damber JE (2004) The expression of thrombospondin-1 in benign prostatic hyperplasia and prostatic intraepithelial neoplasia is decreased in prostate cancer. BJU Int 93(9):1339–1343
Vallbo C, Damber J-E (2005) Thrombospondins, metallo proteases and thrombospondin receptors messenger RNA and protein expression in different tumour sublines of the dunning prostate cancer model. Acta Oncol 44(3):293–298
Lennernas B, Albertsson P, Damber JE, Norrby K (2004) Antiangiogenic effect of metronomic paclitaxel treatment in prostate cancer and non-tumor tissue in the same animals: a quantitative study. APMIS 112(3):201–209
Isaacs JT, Isaacs WB, Feitz WF, Scheres J (1986) Establishment and characterization of seven Dunning rat prostatic cancer cell lines and their use in developing methods for predicting metastatic abilities of prostatic cancers. Prostate 9(3):261–281
Lennernas B, Albertsson P, Lennernas H, Norrby K (2003) Chemotherapy and antiangiogenesis—drug-specific, dose-related effects. Acta Oncol 42(4):294–303
Urakami S, Igawa M, Kikuno N, Yoshino T, Kishi H, Shigeno K et al (2002) Combination chemotherapy with paclitaxel, estramustine and carboplatin for hormone refractory prostate cancer. J Urol 168(6):2444–2450
Albertsson P, Lennernäs B, Norrby K (2005) On metronomic chemotherapy: modulation of angiogenesis mediated by VEGF-A. Acta Oncol (in press)
Yoo GH, Piechocki MP, Ensley JF, Nguyen T, Oliver J, Meng H et al (2002) Docetaxel induced gene expression patterns in head and neck squamous cell carcinoma using cDNA microarray and PowerBlot. Clin Cancer Res 8(12):3910–3921
Dameron KM, Volpert OV, Tainsky MA, Bouck N (1994) The p53 tumor suppressor gene inhibits angiogenesis by stimulating the production of thrombospondin. Cold Spring Harb Symp Quant Biol 59:483–489
Watnick RS, Cheng YN, Rangarajan A, Ince TA, Weinberg RA (2003) Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell 3(3):219–231
Monestiroli S, Mancuso P, Burlini A, Pruneri G, Dell’Agnola C, Gobbi A et al (2001) Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res 61(11):4341–4344
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L et al (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7(11):1194–1201
Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T et al (2005) Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell 7(1):101–111
Capillo M, Mancuso P, Gobbi A, Monestiroli S, Pruneri G, Dell’Agnola C et al (2003) Continuous infusion of endostatin inhibits differentiation, mobilization, and clonogenic potential of endothelial cell progenitors. Clin Cancer Res 9(1):377–382
Ito H, Rovira II, Bloom ML, Takeda K, Ferrans VJ, Quyyumi AA et al (1999) Endothelial progenitor cells as putative targets for angiostatin. Cancer Res 59(23):5875–5877
Pietras K, Hanahan D (2005) A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol 23(5):939–952
Ellis LM (2005) Bevacizumab. Nat Rev Drug Discov Suppl:S8–S9
Acknowledgements
The authors would like to thank the Swedish Cancer Society, the Nilsson, Assar Gabrielsson, Maud and Birger Gustavsson and Sahlgrenska University Hospital Foundations, Sweden for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Damber, JE., Vallbo, C., Albertsson, P. et al. The anti-tumour effect of low-dose continuous chemotherapy may partly be mediated by thrombospondin. Cancer Chemother Pharmacol 58, 354–360 (2006). https://doi.org/10.1007/s00280-005-0163-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0163-8