Abstract
Patient and method
A 42-year-old male patient with relapsing germ-cell cancer was enrolled in a salvage protocol that employed two 4-day courses of CTC high-dose chemotherapy with cyclophosphamide (1,500 mg m−2 day−1), thiotepa (120 mg m−2 day−1), and carboplatin, followed by peripheral blood progenitor cell support. From five days before the start of the second CTC course the patient received phenytoin for generalized epileptic seizures. Blood samples were collected on day 1 of both CTC courses and analyzed for cyclophosphamide and its activated metabolite 4-hydroxycyclophosphamide, and for thiotepa and its main active metabolite tepa.
Results
Exposure (expressed as area under the plasma concentration vs time curve) to 4-hydroxycyclophosphamide and tepa in the second CTC course was increased by 51% and 115%, respectively, compared with the first CTC course, whereas exposure to cyclophosphamide and thiotepa was significantly reduced (67% and 29%, respectively). Because high exposure to 4-hydroxycyclophosphamide and tepa correlates with increased toxicity, the treatment risk of this patient was significantly increased. Therefore doses were reduced on the third day of the second course.
Conclusion
It was concluded that phenytoin significantly induces both cyclophosphamide and thiotepa metabolism, most probably by induction of the cytochrome p450 enzyme system. This potential clinical significant interaction should be taken into account when phenytoin is administered in combination with cyclophosphamide and thiotepa in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cytochrome P450 (CYP) enzyme system is involved in the metabolism of many therapeutically active agents. Alterations in liver CYP enzyme composition and activity may therefore have a major impact on the pharmacokinetics of these compounds and may be responsible for variation in clinical efficacy and toxicity between patients. Because cancer patients are often treated with multiple drug regimens, pharmacokinetic drug–drug interactions resulting in induction or inhibition of CYP enzyme activity often occur. Knowledge of the magnitude and clinical relevance of these drug–drug interactions is therefore important for accurate dosing of chemotherapeutic agents.
Cyclophosphamide and thiotepa are alkylating agents both metabolized by CYP enzymes to active and inactive metabolites. They are often co-administered in high-dose chemotherapy regimens with stem-cell support. The CTC regimen, in which high doses of cyclophosphamide, thiotepa, and carboplatin are administered, is used in the treatment of breast cancer [17] or germ-cell cancer [15, 16]. Cyclophosphamide itself is an inactive prodrug, requiring activation by, primarily, CYP 2B6 to form its activated metabolite 4-hydroxycyclophosphamide [1, 14, 18, 25]. Thiotepa is metabolized by CYP 2B6 and 3A4 iso-enzymes resulting in the formation of the active metabolite tepa [8]. Tepa has a longer elimination half-life than thiotepa but similar pharmacologic properties [6, 22].
The CTC high-dose chemotherapy regimen may be complicated by severe and sometimes life threatening toxicity [7, 15, 19]. This might partly be because of individual differences in exposure to the different compounds in the regimen. We [7] and others [11] have previously established relationships between the pharmacokinetics of cyclophosphamide and thiotepa and organ toxicity in high-dose chemotherapy. Therefore, co-administered agents modifying cyclophosphamide and thiotepa pharmacokinetics may influence treatment outcome. In this manuscript we report major induction of metabolism of both cyclophosphamide and thiotepa by phenytoin, resulting in considerably increased formation of their active metabolites 4-hydroxycyclophosphamide and tepa, respectively.
Patient and method
Case
A 42-year-old male patient (height 167 cm, weight 80 kg) presented with relapsing non-seminomous testis cancer with localizations para-aortal, in the lung, and the bone. The patient was enrolled in a salvage protocol that employed the CTC high-dose chemotherapy regimen with autologous peripheral blood progenitor cell transplantation as described previously [16]. As part of this treatment, he was scheduled to receive two CTC courses with 4 weeks in between.
At the end of December 2003, the patient received his first high-dose CTC course. The regimen consisted of 4 days of chemotherapy with cyclophosphamide (1,500 mg m−2 day−1) as a 1-h infusion, immediately followed by carboplatin (dose calculated on the basis of the Calvert formula, using a target area under the plasma concentration–time curve (AUC) value of 5 mg min−1 mL−1 day−1) as a daily 1 h infusion, and thiotepa (120 mg m−2 day−1) divided over two 30-min infusions (the second daily dose of thiotepa was administered 12 h after the first dose). Mesna (500 mg) was administered six times daily for a total of 36 doses, beginning 1 h before the first cyclophosphamide infusion. Starting 4 days before chemotherapy, the patient prophylactically received antibiotics (ciprofloxacin and fluconazole orally). Furthermore, during the course the patient received anti-emetics (dexamethasone and granisetron), ranitidine, fytomenadion (vitamin K), and folic acid. Full details of the CTC regimen have been published previously [15, 16].
The second CTC course was administered 28 days after the first and did not differ from the first course, except that the patient received phenytoin because of an epileptic seizure that developed 3 weeks after the first CTC course. MRI scans suggested that the seizures were probably attributable to scar tissue after craniotomy. Five days before the start of the second CTC course, the patient started with twice daily 150 mg phenytoin orally.
Pharmacokinetic analysis
For pharmacokinetic analyses during the CTC course, blood samples were withdrawn on the first day of both courses. Samples were collected from a double lumen intravenous catheter inserted in a subclavian vein. Collection took place before the start of the infusions, 30 min after the start of cyclophosphamide infusion (t=30), and at t=60 (end of cyclophosphamide infusion), 90, 120 (end of carboplatin infusion), 150 (end of thiotepa infusion), 180, 210, 280, 390, and 660 min. Analytical methods for determination of plasma concentrations of cyclophosphamide, 4-hydroxycyclophosphamide, thiotepa, tepa, and carboplatin were used as reported previously [4, 24]. A previously published population pharmacokinetic model of thiotepa (and its metabolite tepa) and cyclophosphamide (and its metabolite 4-hydroxycyclophosphamide) was used for calculating the AUC for all compounds using Bayesian analysis [3]. The Committee on the Medical Ethics of the Netherlands Cancer Institute had approved the protocol and written informed consent was obtained from the patient.
Results
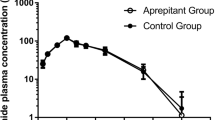
Plasma concentration versus time curves for cyclophosphamide, 4-hydroxycyclophosphamide, thiotepa, and tepa, for both the first and second CTC courses, are shown in Fig. 1. Both cyclophosphamide and thiotepa clearance were increased in the presence of phenytoin, resulting in an increased rate of formation of their respective metabolites 4-hydroxycyclophosphamide and tepa (133% vs 126%, respectively). Exposure to thiotepa was reduced by 29% when phenytoin was co-administered (114 μmol L−1 h vs 81 μmol L−1 h), with a concomitant increase in the tepa AUC of 115% (233 μmol L−1 h vs 501 μmol L−1 h). The plasma exposure to cyclophosphamide decreased by 67% upon co-administration of phenytoin (6,637 μmol L−1 h vs 2,194 μmol L−1 h) with concomitant increase in 4-hydroxycyclophosphamide AUC of 51% (144 μmol L−1 h vs 217 μmol L−1 h). The maximum concentration of 4-hydroxycyclophosphamide was 600% higher when phenytoin was co-administered (1,090 ng mL−1 vs 6,550 ng mL−1). The elimination of 4-hydroxycyclophosphamide was, however, increased, because elimination of this metabolite is formation-rate limited. Plasma levels of carboplatin were similar in courses one and two.
Day-1 plasma concentration versus time curves for A cyclophosphamide (CP; 2,835 mg) and B its metabolite 4-hydroxycyclophosphamide (4OHCP), C thiotepa (115 mg) and D its metabolite tepa, in two high-dose CTC chemotherapy courses, with the course without phenytoin (open circles) and the course with phenytoin co-administration (filled squares)
On the basis of the pharmacokinetic analyses of day one of the second course, the doses of both cyclophosphamide and thiotepa were reduced on days three and four of this course to reach a predefined target exposure (the median exposure in a reference population receiving similar doses) [2]. The cyclophosphamide dose was reduced from 2,835 mg day−1 to 1,500 mg day−1 and the thiotepa dose was reduced from 230 mg day−1 to 140 mg day−1. This adaptation resulted in plasma levels of 4-hydroxycyclophosphamide and tepa within the predefined therapeutic window (data not shown).
Both the first and the second CTC course were uncomplicated and well-tolerated. Mucositis and diarrhea grade I were observed. After stem cell re-infusion, bone marrow recovery was fast.
Suboptimum phenytoin plasma levels of 4–5.5 mg L−1 were measured before, during, and after the second CTC course (optimum 8–18 mg L−1) and therefore the phenytoin dose was increased after the CTC course to twice daily 200 mg.
Discussion
This case shows major induction of cyclophosphamide and thiotepa metabolism by co-administration of phenytoin. Phenytoin is an anticonvulsant drug useful in the treatment of epilepsy. It is also used to reduce brain activity in patients with primary or secondary brain tumors or after brain surgery. Phenytoin is a potent inducer of human CYP 1A2, 2B6 [5, 9, 20], 3A [9, 12, 21, 23] and 2C subfamilies [10]. It is therefore known to lower the efficacy of many drugs, resulting in the need to increase their doses. Phenytoin, and other inducing anti-epileptic agents, have been found to lower the efficacy of antineoplastic treatments [13].
The observed interaction of phenytoin with cyclophosphamide and thiotepa may be explained by induction of CYP enzymes by phenytoin. Of the enzymes involved in cyclophosphamide activation and thiotepa metabolism, as outlined in the introduction, CYP2B6 is the most likely iso-enzyme involved in this interaction. Other authors have demonstrated induction of cyclophosphamide hydroxylation by phenytoin in patients [20]. Patients treated with phenytoin and busulfan in combination with cyclophosphamide in a bone marrow transplantation regimen had cyclophosphamide clearance 112% higher than patients receiving cyclophosphamide with radiotherapy. The half-life of cyclophosphamide was 54% lower and the 4-hydroxycyclophosphamide AUC corrected for cyclophosphamide dose was 48% higher. These findings are in accordance with ours. To our knowledge, no reports have been published on the induction of thiotepa metabolism by phenytoin.
No extreme toxicity was observed in our patient after course one or course two. Timely reduction of the cyclophosphamide and thiotepa dose during the second course, and therefore lowering of total 4-hydroxycyclophosphamide and tepa exposure, may have contributed to the lack of toxicity after the second course.
In conclusion, the drug–drug interaction between phenytoin and both cyclophosphamide and thiotepa results in dramatically higher exposure to the active metabolites 4-hydroxycyclophosphamide and tepa, respectively, and is therefore of potential clinical importance. Because the magnitude of the interaction is not predictable in an individual and may vary interindividually, it is preferable to avoid co-administration of phenytoin with cyclophosphamide and thiotepa therapy. When anti-epileptic therapy is mandatory (for example in brain tumor-related seizures), drugs with no relevant induction of hepatic enzyme systems (valproic acid, gabapentin) should be preferred. When phenytoin administration cannot be avoided, starting doses of cyclophosphamide and thiotepa should be reduced and levels of their active metabolites in plasma should be measured as a guide to further cyclophosphamide and thiotepa dosing.
References
Chang TKH, Weber GF, Crespi CL et al (1993) Differential activation of cyclophosphamide and ifosfamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 53:5629–5637
De Jonge ME, Huitema ADR, Van Dam SM et al (2003) Pharmacokinetically guided dosing of cyclophosphamide, thiotepa and carboplatin in high dose chemotherapy. Proc Am Soc Clin Oncol 22:140 (abstr)
De Jonge ME, Huitema ADR, Rodenhuis S et al (2004) Integrated population pharmacokinetic model of both cyclophosphamide and thiotepa suggesting a mutual drug–drug interaction. J Pharmacokin Pharmacodyn 31:135–156
De Jonge ME, Van Dam SM, Hillebrand MJX et al (2004) Simultaneous quantification of cyclophosphamide, 4-hydroxycyclophosphamide, N,N′,N″-triethylenethiophosphoramide (thiotepa) and N,N′,N″-triethylenephosphoramide (tepa) in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC-MS/MS). J Mass Spectrom 39:233–254
Ducharme MP, Bernstein ML, Granvil et al (1997) Phenytoin-induced alteration in the N-dechloroethylation of ifosfamide stereoisomers. Cancer Chemother Pharmacol 40:531–533
Heideman RL, Cole DE, Balis F et al (1989) Phase I and pharmacokinetic evaluation of thiotepa in the cerebrospinal fluid and plasma of pediatric patients: evidence for dose-dependent plasma clearance of thiotepa. Cancer Res 49:736–741
Huitema ADR, Spaander M, Mathôt RAA et al (2002) Relationship between exposure and toxicity in high-dose chemotherapy with cyclophosphamide, thioTEPA and carboplatin. Ann Oncol 13:374–384
Jacobson PA, Green K, Birnbaum A et al (2002) Cytochrome P450 isozymes 3A4 and 2B6 are involved in the in vitro human metabolism of thiotepa to tepa. Cancer Chemother Pharmacol 49:461–467
LeCluyse EL, Ribadeneira MD, Diamond S et al (1996) Induction of CYP1A, CYP2A, CYP2B, CYP2C, and CYP3A by various drugs and prototypical inducers in primary cultures of human hepatocytes. ISSX Proc 10:196
Nation RL, Evans AM, Mike RW et al (1990) Pharmacokinetics drug interactions with phenytoin. Part I and II. Clin Pharmacokinet 18:37–60 (see also pages 131–150)
Petros WP, Broadwater G, Berry D et al (2002) Association of high-dose cyclophosphamide, cisplatin, and carmustine pharmacokinetics with survival, toxicity, and dosing weight in patients with primary breast cancer. Clin Cancer Res 8:698–705
Pichard L, Fabre I, Fabre G et al (1990) Cyclosporin A drug interactions: screening for inducers and inhibitors of cytochrome P-450 (cyclosporine A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos 18:595–606
Relling MV, Pui CH, Sandlund JT et al (2000) Adverse effects of anticonvulsants on efficacy of chemotherapy for acute lymphoblastic leukemia. Lancet 356:285–290
Ren S, Yang JS, Kalhorn TF et al (1997) Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res 57:4229–4235
Rodenhuis S, Westerman A, Holtkamp MJ et al (1996) Feasibility of multiple courses of high-dose cyclophosphamide, thiotepa, and carboplatin for breast cancer or germ cell cancer. J Clin Oncol 14:1473–1483
Rodenhuis S, De Wit R, De Mulder PHM et al (1999) A multi-center prospective phase II study of high-dose chemotherapy in germ-cell cancer patients relapsing from complete remission. Ann Oncol 10:1467–1473
Rodenhuis S, Bontenbal M, Beex LV et al (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med 349:7–16
Roy P, Yu LJ, Crespi CL et al (1999) Development of a substrate activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos 27:655–666
Schrama JG, Holtkamp MJ, Baars JW et al (2003) Toxicity of the high-dose chemotherapy CTC regimen (cyclophosphamide, thiotepa, carboplatin): the Netherlands Cancer Institute experience. Br J Cancer 88:1831–1838
Slattery JT, Kalhorn TF, McDonald GB et al (1996) Conditioning regimen-dependent disposition of cyclophosphamide and hydroxycyclophosphamide in human marrow transplantation patients. J Clin Oncol 14:1484–1494
Spaans E, Van Den Heuvel MW, Schnabel PG et al (2002) Concomitant use of mirtazapine and phenytoin: a drug–drug interaction study in healthy male subjects. Eur J Clin Pharmacol 58:423–429
Teicher BA, Waxman DJ, Holden SA et al (1989) Evidence for enzymatic activation and oxygen involvement in cytotoxicity and anti-tumor activity of N,N′,N″-triethylenethiophosphoramide. Cancer Res 49:4996–5001
Thummel KE, Shen DD, Podoll T et al (1994) Use of midazolam as a human cytochrome P-450 3A probe. II. Characterization of inter- and intra-individual variability following liver transplantation. J Pharmacol Exp Ther 271:557–566
Van Warmerdam LJC, Van Tellingen O, Maes RAA et al (1995) Validated method for the determination of carboplatin in biological fluids by Zeeman atomic absorption spectrometry. Fresenius J Anal Chem 352:777–781
Yu L, Waxman J (1996) Role of cytochrome P450 in oxazaphosphorine metabolism. Deactivation via N-dechloroethylation and activation via 4-hydroxylation catalyzed by distinct subsets of rat liver cytochromes P450. Drug Metab Dispos 24:1254–1262
Acknowledgment
This work was supported by a grant from the Dutch Cancer Society (project NKI 2001-2420).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Jonge, M.E., Huitema, A.D.R., van Dam, S.M. et al. Significant induction of cyclophosphamide and thiotepa metabolism by phenytoin. Cancer Chemother Pharmacol 55, 507–510 (2005). https://doi.org/10.1007/s00280-004-0922-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0922-y