Abstract
Background
Capecitabine (Xeloda) is a novel, oral fluoropyrimidine carbamate rationally designed to generate 5-fluorouracil (5-FU) preferentially in tumor tissue via a three-step enzymatic cascade.
Purpose
The objective of this study was to compare the pharmacokinetics of capecitabine and its metabolites in Japanese and Caucasian cancer patients.
Methods
The study included 20 Japanese and 24 Caucasian patients with breast cancer. All patients received oral capecitabine 825 mg/m2 twice daily for 14 days, except for study day 1 when only the morning dose was administered. On study days 1 and 14, blood and urine samples were collected after administration of the first dose and at steady state for the evaluation of the pharmacokinetics of capecitabine and its metabolites. The primary pharmacokinetic parameter was AUC0–∞ of 5′-deoxy-5-fluorouridine (5′-DFUR) on day 14. The pharmacokinetic parameters in Japanese and Caucasian patients were compared using an ANOVA with calculation of the 90% confidence interval (CI) for the ratio of the geometric means.
Results
Statistical analysis showed equivalence in the AUC of 5′-DFUR on day 14 with a ratio of 1.01 (90% CI 0.85–1.21). Similarly, no relevant influence of race on the pharmacokinetics of capecitabine, 5′-deoxy-5-fluorocytidine (5′-DFCR), or 5-FU was observed. Systemic exposure to α-fluoro-β-alanine (FBAL) was higher in Caucasian than in Japanese patients. On study day 14, both the AUC and the maximum plasma concentration (Cmax) of FBAL were increased by 47% and 33% in Caucasian patients and Japanese patients, respectively.
Conclusions
No clinically relevant differences in the pharmacokinetics of capecitabine and its key metabolites 5′-DFUR, 5′-DFCR, and 5-FU were found between Japanese and Caucasian patients. Plasma concentrations of FBAL were higher in Caucasian than in Japanese patients but this difference is not clinically relevant as FBAL has no antiproliferative activity and systemic exposure to FBAL does not correlate with the tolerability of capecitabine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethnic differences in the pharmacokinetics of many drugs are well documented [14, 37]. A review of the literature shows that ethnic differences in passive drug absorption have not been reported and only a few cases of differences in gut metabolism/transport or hepatic first-pass metabolism are documented. More commonly, ethnic differences in plasma protein binding occur due to racial differences in plasma concentrations of the binding protein, α1-acid glycoprotein, which affect the volume of distribution and clearance of restrictively cleared drugs. Hepatic metabolism is the pharmacokinetic parameter most commonly showing racial variation. Hepatic metabolism is influenced by ethnic differences in the distribution of polymorphic traits or mutations in the genes encoding hepatic metabolic enzymes, leading to variable expression or activity in different ethnic groups [36]. Ethnic variability in polymorphic traits of drug metabolism, in responsiveness, and in the activity of various drugs has been reviewed by Wood and Zhou [36].
Hepatic metabolism is a major route of elimination of the novel oral fluoropyrimidine carbamate capecitabine (Xeloda; F. Hoffmann-La Roche), which may, therefore, be subject to ethnic differences in pharmacokinetics. Oral capecitabine was rationally designed to preferentially generate 5-FU in tumor tissue and enable chronic dosing that mimics continuous-infusion 5-FU [18]. Following oral administration, capecitabine is rapidly and almost completely absorbed as an intact molecule, and undergoes a three-step enzymatic conversion to 5-FU [26]. Capecitabine and its intermediate metabolites, 5′-DFCR and 5′-DFUR, are not intrinsically cytotoxic. In the final step, the intermediate 5′-DFUR is converted to 5-FU by the enzyme thymidine phosphorylase, which has significantly higher activity in tumor than in normal tissue [18, 19, 26, 31]. Consequently, capecitabine generates 5-FU preferentially at the tumor site, minimizing systemic exposure to 5-FU and potentially enhancing efficacy. Many cytotoxic agents, including the taxanes docetaxel and paclitaxel, further upregulate thymidine phosphorylase in tumor tissue and have shown synergy with capecitabine in preclinical models [29].
Capecitabine is an established therapy for metastatic colorectal and breast cancer. Based on the results of two large phase III trials, capecitabine is approved in more than 80 countries, including the USA and those of the entire European Union (EU), for the first-line treatment of metastatic colorectal cancer [13, 32, 34]. These trials demonstrated that capecitabine achieves significantly superior response rates, equivalent time to disease progression and overall survival, and an improved safety profile compared with i.v. bolus 5-FU/leucovorin (Mayo Clinic regimen). Capecitabine monotherapy is also approved for the treatment of patients with anthracycline- and taxane-pretreated metastatic breast cancer. In four trials conducted in 500 patients with taxane-pretreated metastatic breast cancer, capecitabine achieved consistently high efficacy, with objective response rates in the range 15–25% and median overall survival of approximately 12 months [4, 5, 10, 25]. Most recently, the combination of capecitabine plus docetaxel has gained regulatory approval in numerous countries, including the USA and Canada, and those of the EU, based on the results of a phase III trial [20]. Furthermore, the high antitumor activity and favorable safety profile, particularly the low rate of myelosuppression of oral capecitabine, make it an attractive agent for incorporation into combination regimens, and novel combination regimens incorporating capecitabine are being investigated in numerous studies in patients with gastrointestinal cancer [8, 9, 15, 16, 30, 33], breast cancer [2, 3, 22, 27, 35], and several other tumor types [1, 12, 21, 23].
The recommended single-agent dose of capecitabine is 1250 mg/m2 twice daily (at 12-h intervals), days 1 to 14 followed by a 7-day rest period. In patients with breast or colorectal cancer, the standard single-agent dose of capecitabine is well tolerated, with a low incidence of grade 3/4 adverse events and a particularly low incidence of myelosuppression and alopecia [6, 7].
To enable a comparison of efficacy and safety results from clinical studies performed in Europe/North America and Japan, a study was conducted comparing the pharmacokinetics of capecitabine and its metabolites in Caucasian and Japanese patients. Previous studies have shown that both the AUC and Cmax of 5-FU increase following repeated administration of capecitabine [26]. Therefore, the impact of race on the pharmacokinetics of capecitabine and its metabolites was investigated on study days 1 and 14 (at steady state) following oral administration of capecitabine.
Materials and methods
Study design and patients
This trial was an open-label pharmacokinetic study performed in four Japanese and five European centers. The trial was conducted in full agreement with the principles of the Declaration of Helsinki III. The trial protocol was approved prior to commencement by the Ethical Review Board of the participating institutions. Written informed consent was obtained from each patient before the start of the study. Screening included physical examination, recording of medical history, vital signs, laboratory tests (hematology, blood chemistry, urinalysis), tumor assessment, ECG, and a pregnancy test. Adverse events were monitored continuously throughout the study and for up to 28 days after administration of the last dose. Vital signs and laboratory parameters were assessed on study days 1 and 14, and at follow up (study day 21±2).
Carefully selected exclusion criteria taking into account disease stage, current medical status, and life expectancy of the patients were applied to obtain evaluable clinical and pharmacokinetic data. All patients were ambulatory with histologically/cytologically confirmed advanced or metastatic breast cancer, had a life expectancy of at least 12 weeks, and had no standard treatment options available (Caucasian patients) or had failed previous treatment with docetaxel (Japanese patients).
In the pharmacokinetic phase of this study, a 300-mg tablet formulation was used and all patients received oral capecitabine 825 mg/m2 twice daily. This dose corresponds to the dosing regimen used in Japan during the clinical development of capecitabine (825 mg/m2 twice daily for 21 days, followed by a 7-day rest period). After follow-up assessments, Caucasian patients were allowed to continue capecitabine treatment (at the approved dosing regimen in the EU and USA of 1250 mg/m2 twice daily for 2 weeks followed by 1 week rest) for up to 54 weeks and Japanese patients continued their treatment with capecitabine as this pharmacokinetic assessment was part of a phase II study.
On study days 1 and 14, patients attended the hospital after having fasted overnight from midnight. Patients were dosed under fasted conditions and took the medication with 200 ml of water. A standard breakfast was served 2 h after dosing.
Blood and urine sampling
On study day 1, the evening dose was not administered in order to quantify urinary recovery of capecitabine and its metabolites during a 24-h collection interval. On study days 1 and 14, 5-ml blood samples were collected at baseline (predose) and 0.25, 0.5, 1, 2, 3, 4, 5, 6, 8, and 11 (±1) hours after dosing using Vacutainers containing EDTA as anticoagulant. Blood samples were centrifuged at 1500 g and 4°C for 10 min, and the supernatant plasma was removed and stored in plastic tubes at −20°C until analysis.
Urine was collected and pooled during the following time intervals: predose, 0–11 h, and 11–24 h on study day 1 and 0–11 h on study day 14. At the end of each interval, the total volume and the pH of the urine were recorded, and a 15-ml aliquot was removed and stored at −20°C until drugs were assayed.
Drug assay
Plasma and urine concentrations of capecitabine and its metabolites were determined by a validated liquid chromatography with mass-spectrometry detection (LC/MS-MS) [26].
Pharmacokinetic parameters
Pharmacokinetic parameters were assessed by standard non-compartmental analysis [11], using SAS version 6.12 for Windows/NT [28]. The pharmacokinetic parameters of capecitabine and its metabolites (5′-DFCR, 5′-DFUR, 5-FU, and FBAL) were estimated for each patient from the concentration-time data on study days 1 and 14. Maximum plasma concentration (Cmax) and the time to reach this value (tmax) were determined from the highest observed concentration and the time at which it occurred. Apparent half-life, t1/2, was estimated from ln2/λ, where the apparent rate constant of elimination, λ, was estimated by linear regression on the logarithm of the plasma concentration versus time data. The area under the plasma concentration time curve from time 0 to infinity (AUC0–∞) was estimated from the sum of AUC0–t and Ctlast/λ, where AUC0–t is the area under the curve from time 0 to the last sampling time (tlast) at which a concentration above the limit of quantification was measured (Ctlast). AUC0–t was estimated using the linear trapezoidal rule.
Percentage of dose recovered in urine as capecitabine or one of its metabolites was calculated based on dose, urinary concentration, and volume of urine collected during the intervals on study days 1 and 14 described above.
Statistical analysis
The primary pharmacokinetic parameter compared in Caucasian and Japanese patients was AUC0–∞ of 5′-DFUR on study day 14. All other pharmacokinetic parameters, listed in Tables 1 and 2, were considered secondary parameters.
The sample size of 20 patients per group was calculated based on the coefficient of variation (CV%) of the primary pharmacokinetic parameter AUC0-∞ of 5′-DFUR estimated in previous studies [26]. The statistical analysis was performed using an analysis of variance with race as a factor and after logarithmic transformation of the pharmacokinetic variable. The race effect ratio (ratios of the geometric means) and associated confidence limits on the untransformed scale were calculated by exponentiation of the least squares mean differences and the confidence limits obtained for the transformed values. Equivalence was concluded if the 90% confidence interval of the ratio of the means was within the range 0.70–1.43. This interval was selected based on the interpatient variability in the pharmacokinetics of capecitabine and its metabolites observed in previous studies and on current practice in the field of pharmacokinetics when testing for equivalence.
In addition to the primary pharmacokinetic parameter, the secondary parameters AUC, Cmax , t1/2, and tmax of capecitabine, 5′-DFCR, 5-FU, and FBAL were analyzed on study days 1 and 14 in an exploratory manner. For AUC, Cmax, and t1/2, the same statistical test as for AUC of 5′-DFUR was used. The non-parametric, two-sample Wilcoxon test was used to compare tmax as this parameter always corresponds to one of the predetermined sampling times.
Results
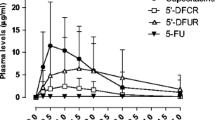
Included in the study were 20 female Japanese patients with advanced/metastatic breast cancer (age 42–68 years, weight 39–66 kg, BSA 1.3–1.7 m2, creatinine clearance 68–145 ml/min) and 24 female Caucasian patients (age 33–76 years, weight 48–91 kg, BSA 1.4–2.0 m2, creatinine clearance 35–138 ml/min). Descriptive statistics for the pharmacokinetic parameters of capecitabine and its metabolites, estimated on study days 1 and 14, are presented in Tables 1 and 2, respectively. Mean plasma concentration profiles of 5′-DFUR and of FBAL on study days 1 and 14 are shown in Figs. 1, 2, 3 and 4, respectively. Urinary recoveries of capecitabine and its metabolites are presented in Table 3.
Influence of race on the pharmacokinetics of 5′-DFUR
Plasma concentrations of 5′-DFUR on days 1 and 14 of treatment were similar in Japanese and Caucasian patients (Figs. 1 and 2). The primary pharmacokinetic parameter, AUC0–∞ of 5′-DFUR on day 14 was also similar between the two races with a ratio of 1.01 and a 90% confidence interval of 0.85–1.21 (Table 2). Since the 90% confidence interval was within the predefined limits of 0.70–1.43, equivalence between the two groups was concluded for AUC0–∞ of 5′-DFUR. On day 1, the AUC0–∞ of 5′-DFUR was 18% lower in Caucasian patients than in Japanese patients (Table 1). However, the 90% confidence interval of 0.69–0.98 was very close to but outside the predefined limits, but this very small deviation is not considered clinically relevant. On day 1, Cmax was 12% lower in Caucasian patients than in Japanese patients while on day 14, this parameter was 12% higher in the Caucasian patients. In both groups, high interpatient variability was observed for Cmax (CV% from 44% to 61%). For both study days, equivalence could not be concluded for Cmax of 5′-DFUR. There was equivalence in the elimination half-life of 5′-DFUR between the two races on both days. Furthermore, no statistically significant differences (α level 5%) in tmax between the two races were revealed by the exploratory statistical analysis using the two-sample Wilcoxon test (P=0.162 on day 1, P=0.268 on day 14).
Influence of race on the pharmacokinetics of capecitabine
On both study days, a high interpatient variability was observed in the Cmax of capecitabine in both Japanese and Caucasian patients. In addition, high interpatient variability in the capecitabine AUC was also apparent in Caucasian patients (Tables 1 and 2). As there were no major differences observed on study day 1 in the pharmacokinetic parameters for capecitabine (AUC0–∞, Cmax, and t1/2) in Japanese and Caucasian patients, equivalence was concluded for AUC and t1/2. On day 14, higher values for AUC0–∞ (+23%), Cmax (+41%), and t1/2 (+28%) were observed in Caucasian patients compared with Japanese patients. Therefore, the AUC0–∞ and Cmax of capecitabine were not equivalent in Caucasian and Japanese patients (Table 2), although the elimination half-life was equivalent in the two groups. No statistically significant differences in tmax were observed between the two races on either study day (P=0.083 on day 1, P=0.096 on day 14).
Influence of race on the pharmacokinetics of 5′-DFCR
There were no relevant differences in the pharmacokinetic parameters of 5′-DFCR on days 1 and 14 between Japanese and Caucasian patients (Tables 1 and 2). For all parameters, with the exception of Cmax on day 14, the 90% confidence intervals were within the predefined limits for equivalence. On day 14, Cmax was 14% higher in Caucasian patients and equivalence could not be concluded for this parameter. However, due to high intersubject variability of 79% (Table 2), the study was not powered to show equivalence with regard to Cmax. No statistically significant differences in tmax between the two races were evident on either study day (P=0.066 on day 1, P=0.132 on day 14).
Influence of race on the pharmacokinetics of 5-FU
The pharmacokinetic parameters AUC0–∞ and t1/2 of 5-FU, the metabolite with cytotoxic activity, were shown to be equivalent in Japanese and Caucasian patients on both study days. Equivalence could not be concluded for Cmax which showed high interpatient variability on both days and in both populations (Tables 1 and 2). In Caucasian patients, the Cmax of 5-FU was 11% lower on day 1 but 26% higher on day 14 compared with Japanese patients. No evidence for a difference in the tmax of 5-FU between the two races was observed on either study day (P=0.191 on day 1, P=0.332 on day 14).
Influence of race on the pharmacokinetics of FBAL
Pharmacokinetic profiles showed that the plasma concentrations of FBAL were higher in Caucasian patients than in Japanese patients on both study days (Figs. 3 and 4). In Caucasian patients, the AUC0–∞ of FBAL was increased by 52% on day 1 and by 47% on day 14 and the increase in Cmax reached 39% on day 1 and 33% on day 14 (Tables 1 and 2). In contrast, no evidence for a difference in tmax between the two races was observed on either study day (P=0.918 on day 1, P=0.453 on day 14).
Urinary excretion of capecitabine and its metabolites
Urinary recoveries of capecitabine and its metabolites on study days 1 and 14 are presented in Table 3. Recoveries of capecitabine and 5-FU were similar in Caucasian and Japanese patients on days 1 and 14. However, recoveries of 5′-DFUR and 5′-DFCR were lower in Caucasian than in Japanese patients. Urinary recoveries of α-fluoro-β-ureido propionic acid and FBAL were similar on day 1 in the two races, but on day 14, the amounts of these two metabolites recovered in urine were higher in the Caucasian patients.
Overall, the total urinary excretion of capecitabine plus its metabolites was lower in the Caucasian patients on day 1 but similar in both races on day 14. The intersubject variability in urinary recovery of intact drug and metabolites was very high in both races and on both study days, most likely owing to the difficulty of achieving complete collection of urine in cancer patients.
Discussion
The objective of this study was to compare the pharmacokinetics of capecitabine and its metabolites in Japanese and Caucasian patients with cancer. Capecitabine is undergoing extensive clinical development in Japan, Europe, and North America. Comparison of efficacy and safety results in Caucasian and Japanese patients requires evaluation of the influence of race on the pharmacokinetics of capecitabine and its metabolites. In the present study, comparison of safety between the two races was not an objective as the capecitabine dose in the Caucasian group was changed from 825 mg/m2 to the recommended dose of 1250 mg/m2 twice daily after the first 3 weeks of treatment.
The primary pharmacokinetic parameter in the present study, the AUC0–∞ of 5′-DFUR on day 14, was chosen because 5′-DFUR is the direct precursor of 5-FU and a previous study has shown a positive correlation between the AUC0–∞ of 5′-DFUR on day 14 of treatment and safety (i.e., the incidence of grade 3 or 4 treatment-related adverse events) [24]. All patients evaluated in this prospective study had advanced/metastatic breast cancer. Blood sampling times, dosing under fasting conditions, pharmacokinetic assays, and tablet formulation were identical in the Caucasian and Japanese cohorts. Demographic data showed that the two groups were similar in terms of age (mean 55 and 54 years in the Japanese and Caucasian patients, respectively) but not in terms of body size, with Japanese patients being smaller (mean BSA 1.46 and 1.76 m2 in the Japanese and Caucasian patients, respectively). As the capecitabine dose was adjusted according to the BSA of each patient, the difference in body size between the two groups would not have affected the conclusions drawn from the study.
The primary pharmacokinetic parameter, the AUC0–∞ of 5′-DFUR on day 14, was equivalent in the Japanese and Caucasian patients. Systemic exposure (as revealed by AUC and Cmax) to capecitabine, 5′-DFCR, and 5-FU was similar in the Japanese and Caucasian patients, although equivalence was not always demonstrated. High interpatient variability, especially in the Cmax of capecitabine and its metabolites, may have contributed to the minor deviations observed. Evaluation of a larger number of patients may have enabled statistical equivalence in these parameters to be demonstrated between the two populations.
Systemic exposure to the metabolite FBAL was significantly higher (by approximately 50%) in the Caucasian than in the Japanese patients. However, FBAL is not intrinsically cytotoxic and a previous study has shown that systemic exposure to FBAL does not correlate with safety, strongly suggesting that FBAL itself is unlikely to cause adverse events [24]. FBAL is excreted mainly via the kidneys and therefore its elimination rate is related to creatinine clearance. A possible explanation for the 50% difference in FBAL AUC could be the lower creatinine clearance observed in Caucasian patients than in Japanese patients (mean 81.4 ml/min and 97.1 ml/min, respectively). Ethnic differences in renal clearance were also reflected by the longer elimination half-life of FBAL observed on both study days in the Caucasian patients. However, the difference in creatinine clearance (16% lower in the Caucasian patients than in the Japanese patients) is too small to explain the magnitude of the difference in AUC of FBAL between the two groups.
A high interpatient variability in the systemic exposure to capecitabine and its metabolites was observed (Tables 1 and 2). There were no apparent and consistent differences in this variability between the Caucasian and Japanese patients, or between day 1 and day 14. As expected, the interpatient variability was higher for Cmax than for AUC. Oral administration, along with the extensive metabolism of capecitabine and most of its metabolites contributes to high interpatient variability [25]. Similar variability in the pharmacokinetics of capecitabine has been observed in other clinical pharmacokinetic studies [25] and the results from the present study are therefore consistent with previous findings.
The dose of 825 mg/m2 twice daily was selected in this study because it corresponds to the dosing regimen used in Japan during the clinical development of capecitabine (825 mg/m2 twice daily for 3 weeks, followed by 1 week rest). This dose is lower than the approved dosing regimen in countries other than Japan (1250 mg/m2 twice daily for 2 weeks followed by 1 week rest). This difference in doses raises the issue of whether the outcome of the study would have been the same if the dose of 1250 mg/m2 had been used. In theory, it cannot be totally excluded that the race effect could be dose-dependent. However, this hypothesis is very unlikely as the enzymes responsible for the metabolism of capecitabine and its metabolites would not get close to saturation at a dose of 1250 mg/m2 in either Japanese or Caucasian patients. In phase I studies, dose proportionality for 5′-DFUR was observed at doses up to 1500 and 1250 mg/m2 in Caucasian and Japanese patients, respectively [17].
In conclusion, no clinically relevant differences in the pharmacokinetics of capecitabine, 5′-DFUR, 5′-DFCR, or 5-FU were found between Japanese and Caucasian patients. For FBAL, plasma concentrations were higher in Caucasian patients than in Japanese patients but this difference is not clinically relevant as FBAL has no antiproliferative activity and systemic exposure to FBAL does not correlate with the safety of capecitabine.
Abbreviations
- AUC:
-
Area under the plasma concentration versus time curve
- BSA:
-
Body surface area
- Cmax :
-
Maximum plasma concentration
- 5′-DFCR:
-
5′-Deoxy-5-fluorocytidine
- 5′-DFUR:
-
5′-Deoxy-5-fluorouridine
- FBAL:
-
α-Fluoro-β-alanine
- 5-FU:
-
5-Fluorouracil
- t1/2 :
-
Elimination half-life
- tmax :
-
Time to reach maximum plasma concentration
References
Akhtar SU, Mathew P, Lin J, et al (2001) Phase II trial of capecitabine and carboplatin in patients with newly diagnosed, advanced non-small cell carcinoma of the lung (NSCLC) (abstract 2862). Proc Am Soc Clin Oncol 20:278b
Bangemann N, Kuhle A, Ebert A, et al (2000) Capecitabine combined with trastuzumab in the therapy of intensively pretreated HER2-overexpressing metastatic breast cancer (MBC) (abstract 653). Ann Oncol 11 [Suppl 4]:143
Biganzoli L, Bonnefoi H, Mauriac L, et al (2001) Cyclophosphamide (C)–epirubicine (E)–capecitabine (X) combination, CEX: a safe and active regimen in the treatment of locally advanced/inflammatory (LA/I) or large operable (LO) breast cancer (BC). An EORTC-IDBBC study (abstract 539). Eur J Cancer 37 [Suppl 6]:146
Blum JL (2001) The role of capecitabine, an oral enzymatically activated fluoropyrimidine, in the treatment of metastatic breast cancer. Oncologist 6:56–64
Blum JL, Jones SE, Buzdar AU, et al (1999) Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17:485–493
Blum JL, Dieras V, Lo Russo PM, et al (2001) Multicenter, phase II study of oral capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 92:1759–1768
Cassidy J, Twelves C, Van Cutsem E, et al (2002) First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with i.v. 5-fluorouracil (5-FU)/leucovorin. Ann Oncol 13:566–575
Dunst J, Reese T, Sutter T, et al (2002) Phase I trial evaluating the combination of radiotherapy and capecitabine in rectal cancer. J Clin Oncol 20:3983–3991
Evans TRJ, Paul J, McInnes A, et al (2001) Final results of a phase 1 and PK study of capecitabine (XEL) in combination with epirubicin (E) and cisplatin (C) [ECC] in patients with advanced oesophagogastric (OG) adenocarcinoma (abstract 1156). Eur J Cancer 37 [Suppl 6]:312
Fumoleau P, Largillier R, Trillet-Lenoir V, et al (2001) Phase II study of capecitabine (Xeloda(r)) in pts with advanced breast cancer (ABC), previously treated with anthracyclines and taxanes (abstract 435). Breast Cancer Res Treat 69:285
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker, New York
Hess V, Borner M, Morant R, et al (2001) Gemcitabine (GEM) and capecitabine (CAP) for advanced pancreatic carcinoma. A phase I/II trial (abstract 1167). Eur J Cancer 37 [Suppl 6]:315
Hoff PM, Ansari R, Batist G, et al (2001) Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 19:2282–2292
Johnson JA (2000) Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. Int J Clin Pharmacol Ther 36:275–281
Kerr DJ, Ten Bokkel Huinink WW, Bakker J, et al (2001) CPT-11 in combination with capecitabine as first line chemotherapy for metastatic colorectal cancer (MCRC): preliminary results of a phase I/II study (abstract 1093). Eur J Cancer 37 [Suppl 6]:296
Kim T, Ahn J, Lee J, et al (2001) A phase II trial of capecitabine (X) and cisplatin (P) in previously untreated advanced gastric cancer (AGC) (abstract 662). Proc Am Soc Clin Oncol 20:166a
Mackean MJ, Planting AS Th, Twelves C, et al. (1998) A phase I and pharmacologic study of intermittent twice daily oral therapy with capecitabine in patients with advanced and /or metastatic cancer. J Clin Oncol 16:2977–2985
Miwa M, Ura M, Nishida M, et al (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumors by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1281
Mori K, Hasegawa M, Nishida M, et al (2000) Expression levels of thymidine phosphorylase and dihydropyrimidine dehydrogenase in various human tumor tissues. Int J Oncol 17:33–38
O'Shaughnessy J, Miles D, Vukelja S, et al (2002) Superior survival with docetaxel/capecitabine combination therapy in anthracycline-pretreated patients: phase III trial results. J Clin Oncol 20:2812–2823
Övermann K, Buer J, Hoffmann R, et al (2000) Capecitabine in the treatment of metastatic renal cell carcinoma. Br J Cancer 83:583–587
Pagani O, Sessa C, Longhi S, et al (2001) Dose-finding study of docetaxel (T) and doxorubicin (A) day 1 and 8 plus capecitabine (X) day 1 to 14 (TAX) as first line treatment in advanced breast cancer (ABC) (abstract 711). Eur J Cancer 37 [Suppl 6]:194
Pivot X, Chamorey E, Guardiola E, et al (2001) Phase I and pharmacokinetic study of capecitabine and cisplatin in head and neck cancer patients (abstract 260). Eur J Cancer 37 [Suppl 6]:73
Poole C, Gardiner J, Twelves C, et al (2002) Effect of renal impairment on the pharmacokinetics and tolerability of capecitabine (Xeloda) in cancer patients. Cancer Chemother Pharmacol 49:225–234
Reichardt P, von Mickwitz G, Luck HJ, et al (2001) Capecitabine: the new standard in metastatic breast cancer failing anthracycline- and taxane-containing chemotherapy? Mature results of a large multicenter phase II trial (abstract 699). Eur J Cancer 37 [Suppl 6]:191
Reigner B, Blesch K, Weidekamm E (2001) Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet 40:85–104
Rosso R, Del Mastro L, Durando A, et al (2001) Capecitabine in association with epirubicin and docetaxel as a first-line treatment in advanced breast cancer. A multicenter phase II study (abstract 353). Breast Cancer Res Treat 69:270
SAS Institute (1993) SAS Companion for the Microsoft Windows environment, version 6, 1st edn. SAS Institute, Cary, NC
Sawada N, Ishikawa T, Fukase Y, et al (1998) Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res 4:1013–1019
Schleucher N, Tewes M, Achterrath W, et al (2001) Extended phase I study of capecitabine and weekly irinotecan as first-line chemotherapy in metastatic colorectal cancer (abstract 1073). Eur J Cancer 37 [Suppl 6]:290
Schüller J, Cassidy J, Dumont E, et al (2000) Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 45:291–297
Twelves C (2002) Capecitabine as first-line treatment in colorectal cancer: pooled data from two large, phase III trials. Eur J Cancer 38 [Suppl 2]:S15–S20
Twelves C, Butts C, Cassidy J, et al (2001) Capecitabine in combination with oxaliplatin as first line therapy for patients (pts) with advanced or metastatic colorectal cancer (ACRC): preliminary results of an international multicenter phase II study (abstract 1005). Eur J Cancer 37 [Suppl 6]:272
Van Cutsem E, Twelves C, Cassidy J, et al (2001) Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19:4097–4106
Venturini M, Del Mastro L, Garrone O, et al (2002) Phase I, dose-finding study of capecitabine in combination with docetaxel and epirubicin as first-line chemotherapy for advanced breast cancer. Ann Oncol 13:546–552
Wood AJ, Zhou HH (1991) Ethnic differences in drug disposition and responsiveness. Clin Pharmacokinet 20:350–373
Zhou HH, Liu ZQ (2000) Ethnic differences in drug metabolism. Clin Chem Lab Med 38:899–903
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reigner, B., Watanabe, T., Schüller, J. et al. Pharmacokinetics of capecitabine (Xeloda) in Japanese and Caucasian patients with breast cancer. Cancer Chemother Pharmacol 52, 193–201 (2003). https://doi.org/10.1007/s00280-003-0642-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0642-8