Abstract
Purpose
Docetaxel is a semisynthetic taxane derived from the needles of the European yew (Taxus baccata) and it is an important chemotherapeutic agent in the treatment of recurrent ovarian, breast and non-small-cell lung cancers. Traditional dosing regimens with docetaxel involve doses of 60–100 mg/m2 by infusion every 3 weeks. Now weekly low-dose (30–36 mg/m2) regimens are being evaluated in phase I trials. Such low-dose studies require a more sensitive, specific and rapid assay of docetaxel in biological fluids for the determination of pharmacokinetic parameters. Because docetaxel is primarily metabolized by CYP3A4 and is highly protein-bound in the plasma, there is potential for drug-drug interactions and high interpatient variability in pharmacokinetics. Therefore, pharmacokinetic studies are an important component to understanding the therapeutic variability of docetaxel-containing chemotherapeutic regimens.
Methods
To this end, we developed an analytical assay for docetaxel based upon tandem LCMS and paclitaxel as an internal standard. The sensitivity of the new assay allowed us to monitor plasma levels of docetaxel out to 48 h after the end of the infusion in patients enrolled in a phase I trial of exisulind (orally, twice daily) receiving weekly docetaxel doses of 30 or 36 mg/m2 where plasma docetaxel levels are below the lower limit of quantitation for traditional HPLC/UV-based assays at later time-points.
Results
The inclusion of the 48-h time-point had significant effects on the calculated pharmacokinetic parameters when using either a three-compartment or non-compartmental analysis. The terminal half-life was significantly increased when the 48-h time-point was included in the pharmacokinetic analysis, and the use of model parameters derived with the inclusion of the 48-h time-point were able to more accurately predict plasma levels at later times.
Conclusions
The results reflect the importance of accurate and sensitive analytical methods for the determination of pharmacokinetic parameters and the effect of this later time-point on docetaxel pharmacokinetic modeling. Further, with the increased use of weekly docetaxel in combination with other agents, the inclusion of these later sampling time-points and sensitive methods for drug level determinations are important components in the description of pharmacokinetic drug interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The taxanes (docetaxel and paclitaxel, Fig. 1) are very effective anticancer agents used against a broad range of human cancers [13]. Docetaxel is a semisynthetic taxane that is prepared from a non-toxic precursor compound (10-deacetyl baccatin II) that is extracted from needles of the European yew tree. Paclitaxel and docetaxel have the same mechanism of action that involves binding to microtubules and inhibiting depolymerization through the stabilization of the polymer [22]. In vitro studies have shown that docetaxel has an approximately twofold higher affinity than paclitaxel as an inhibitor of microtubule depolymerization, and this translates to increased potency to tumor cells in tissue culture as well as in vivo [28].
The taxanes are administered to patients via parenteral routes, with infusion times ranging from 1 to 24 h. Studies suggest that efficacy is not different when comparing shorter versus longer infusion times [25], and thus in most clinical protocols taxane is infused over 1 to 3 h. Paclitaxel is given at doses in the range 100–200 mg/m2 every 21 days, and docetaxel at doses in the range 50–100 mg/m2. More recently, weekly treatment protocols for paclitaxel and docetaxel have been evaluated at doses of 80–100 mg/m2 and 30–40 mg/m2, respectively [11, 24]. Clinical studies with docetaxel in patients with metastatic breast cancer have shown that weekly protocols are equally as effective as higher-dose treatments every 21 days with decreased toxicity seen with the weekly treatments [2]. The increased therapeutic index seen with this protocol has led to a number of studies testing the safety and efficacy of weekly taxane dosing in a number of combinations with other agents [1, 5, 6, 17, 18, 23, 27].
One potential problem with the use of lower, more frequent doses of taxanes is the difficulty in assessing plasma drug levels over a time frame necessary for the accurate determination of pharmacokinetic parameters. The standard method for taxane analysis in biological matrices is by HPLC with UV detection [7, 20, 32], and tandem LCMS assays have been developed for paclitaxel [3, 15, 21, 30]. The lower limit of quantitation of HPLC with UV detection assays for taxane analysis has been reported to be 5–10 ng/ml in plasma [7, 20, 32], and the use of these assays in our laboratory has given similar lower levels of quantitation. With the limitation of HPLC/UV-based assay sensitivity, two of eight docetaxel pharmacokinetic profiles at a dose of 30 mg/m2 in these studies were below the limit of detection 8 h after dosing and eight of eight 24 h after dosing. Pharmacokinetic parameters calculated using these incomplete samplings are less accurate than those calculated with full time-course samplings and can lead to the misrepresentation of terminal elimination phases.
Although later time-points may have little effect on the calculation of many pharmacokinetic parameters associated with total drug exposure (AUC, CL) for drugs that have high volumes of distribution, these time-points can have profound effects on the estimation of drug levels at extended time-points. These estimates may be very important pharmacokinetic considerations if the maintenance of drug levels above a given concentration is an important indicator of efficacy. Correlations have been found between some toxic effects of the taxanes and the time that plasma levels are above certain concentrations [9, 29], and the use of this metric may be more important for therapeutic prediction in lower-dose protocols in which inhibition of tumor vascular endothelial cell proliferation may play a role in the antitumor activity.
For the more accurate determination of docetaxel pharmacokinetics we have developed a sensitive, tandem LCMS-based assay. The use of this assay for docetaxel analysis allows the measurement of docetaxel in the plasma of cancer patients receiving weekly doses of 30–36 mg/m2 at times to at least 48 h after dosing. In the studies presented here we examined the effect of the inclusion of these later time-points on the calculation of docetaxel pharmacokinetic parameters using compartmental and non-compartmental analysis of the data. The results from these studies suggest that the inclusion of these later time-points can have a significant effect on the determination of the terminal half-life of docetaxel in humans and this can have a profound effect on the prediction of docetaxel plasma levels at extended time-points.
Materials and methods
Chemicals and reagents
Docetaxel was purchased from LKT Laboratories (St. Paul, Minn.) and paclitaxel, which was used as an internal standard, was purchased from Sigma Chemical Co. (St. Louis, Mo.). All other reagents were of analytical grade and were purchased from commercial suppliers.
Study design
Patients with histologically documented solid malignancies refractory to standard therapy or for whom no effective therapy existed were eligible for this study. Other eligibility criteria included: (1) age ≥18 years; (2) Southwestern Oncology Group (SWOG) performance status of ≤1; (3) no prescription or over-the-counter NSAIDs for the 2 weeks prior (low-dose prophylactic aspirin was allowed); (4) predicted life expectancy ≥3 months; (5) no chemotherapy or investigational agents within 4 weeks of study entry or 6 weeks for nitrosoureas or mitomycin C; (6) adequate hematopoietic function (absolute neutrophil count ≥1500/μl, hemoglobin ≥9.0 g/dl, platelet count >100,000/μl), hepatic function (total bilirubin not more than the institutional upper limit of normal, AST and ALT not more than 2.5 times the ULN and alkaline phosphatase not more than the ULN, or alkaline phosphatase less than four times the ULN if transaminases not more than the ULN, or transaminases not more than 1.5 times the ULN and 2.5 times the ULN), and renal function (creatinine concentrations not more than the ULN); (7) no radiation therapy for the prior 2 weeks; and (8) resolution of all previous therapy-related toxicity. Patients with uncontrolled brain metastases (rapidly evolving neurological symptoms, or metastases that needed specific treatment prior to systemic chemotherapy) and those with significant medical conditions (e.g. uncontrolled hypertension, heart disease, or diabetes mellitus) were excluded. Female patients of child-bearing age were required to have a negative pregnancy test prior to study entry and were required to be on adequate birth control while on study. Informed consent was obtained according to federal and institutional guidelines
Patients were enrolled in a phase I study of weekly docetaxel (30–36 mg/m2) in combination with oral exisulind (150–250 mg twice daily). The docetaxel plasma time-course samples used in the studies presented here were from day 1 of cycle 1 prior to patients starting on oral exisulind. Plasma samples were collected prior to starting drug infusion (baseline), at the end of a 1-h drug infusion, and 5 min, 10 min, 20 min, 30 min, 1 h, 2 h, 4 h, 8 h, 24 h and 48 h after the end of infusion. Collected plasma samples were stored at −80°C prior to extraction and analysis.
Analysis of docetaxel
Docetaxel analysis in human plasma was done by a tandem LCMS method developed in our laboratory. The accuracy, precision, reproducibility and development of this assay are described in a manuscript currently under review for publication (Long et al., submitted for publication). Plasma (1 ml) was spiked with 25 μl 1 μM paclitaxel as an internal standard. Samples and standards were centrifuged for 5 min at 2000 g, and the supernatants removed and mixed with 500 μl 50% acetonitrile. Sample extraction was then carried out using a Bond Elut C2 solid-phase extraction column preconditioned with 1 ml methanol followed by 1 ml water. Samples were placed on the column, washed with 3 ml water, and eluted with 1 ml methanol. Samples were then evaporated to dryness and resuspended in mobile phase (90% acetonitrile in 10 mM ammonium acetate) for LC/MS/MS analysis. Analyses were performed with a PE Sciex API-3000 with a turbo ionspray source. The LC system consisted of a 50 mm C-18 column (2 mm i.d.) with an isocratic mobile phase. The flow rate was 200 μl/min and the injection volume 20 μl. The instrument was operated in SRM mode (positive ion), monitoring the ion transitions from m/z 808→226 (docetaxel) and m/z 854→286 (internal standard). The peaks coeluted at 1.6 min with a total analysis time of 4 min. The docetaxel LC/MS/MS assay was linear over the range of 0.25–1000 nM with a lower limit of quantitation (signal to noise ratio of 5) of 0.25 nM (i.e. 4.04 pg injected) in plasma. The accuracy of the docetaxel assay was determined by preparation of standard plasma samples at 7.5, 25 and 50 nM. The accuracy and precision (%RSD) observed were 94.4±3.8% at 7.5 nM, 97.3±2.0% at 25 nM and 98.9±2.0% at 50 nM. Docetaxel concentrations in plasma were calculated based on a standard curve of docetaxel in blank pooled human plasma with the internal standard paclitaxel.
Pharmacokinetic modeling
Pharmacokinetic parameters were calculated from plasma concentration versus time data using a three-compartment model with i.v. infusion (Fig. 2) and by non-compartmental analysis. Compartmental modeling and calculation of three-compartment-based pharmacokinetic parameters were done using WinNonlin version 3.0 with uniform weighting for least squares minimization (Pharsight Corporation, Mountain View, Calif.). Calculation of non-compartmental-based pharmacokinetic parameters and non-compartmental modeling was carried out using Microsoft Excel with standard equations for non-compartmental and system analysis [33].
Data analysis
The predictive capabilities of the compartmental and non-compartmental models were assessed by calculating the median absolute performance error (MAPE%) and the median performance error (MPE%) [26]. The performance errors were calculated as the difference between the measured values and the predicted values normalized to the predicted value as shown in Eq. 1 [10]:
The MAPE%, which is a measure of the accuracy of the prediction, was calculated as shown in Eq. 2 where n is the total number of samples for that time-point:
The MPE%, which is a measure of the bias of the prediction, was calculated as:
Statistical analysis to compare the differences between average pharmacokinetic parameters calculated with and without the inclusion of the 48-h time-point were done by one-way ANOVA using the Tukey post-test for pairwise comparison. Statistical analyses were carried out using Sigma Stat version 2.03 (SPSS, Chicago, Ill.).
Results
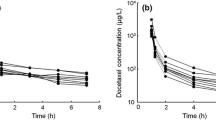
The pharmacokinetics of docetaxel in human plasma could be described using a three-compartment model with constant rate i.v. infusion as shown in Fig. 3 with or without the inclusion of a 48-h time-point, and these data are summarized in Table 1. It is important to note that the addition of later sampling time-points still allowed docetaxel plasma pharmacokinetics to be well described by a three-compartment model since studies with doxorubicin [16] have shown that the addition of later sampling time-points can alter the model structure that describes the data for this drug. The average pharmacokinetic values calculated without the 48-h time-point were consistent with published data of the clinical pharmacokinetics of docetaxel when given as a 1- to 2-h infusion. Summarized clinical data for docetaxel in humans indicate alpha and beta half-lives of 4.5 and 38.3 min, respectively, and a gamma half-life of 12.2 h [4]. We obtained alpha and beta half-lives of 4.3±1.5 and 43.8±21.0 min, respectively, and a gamma half-life of 15.7±7.4 h when plasma time-course data were modeled without the 48-h time-point. When the 48-h time-point was included, the alpha and beta half-lives were calculated to be 4.7±1.9 and 52.8±28.8 min, respectively, and the gamma half-life 25.4±20.1 h. This increase in gamma half-life calculated with the 48-h time-point included was reflected in changes in other pharmacokinetic parameters as shown in Table 1.
Docetaxel plasma time course data fitted to a three-compartment model. The solid line represents the three-compartment model simulation using constants calculated with the 48-h time-point. The dashed line represents the three-compartment model simulation using constants calculated without the 48-h time-point
The average parameters derived from the three-compartment fit with and without the inclusion of the 48-h time-point were used with the equations shown in Fig. 2A and C to derive predicted concentrations based on these values for comparison with the actual values as a measure of fit. The median (MPE%) and absolute median (MAPE%) prediction errors for each of the collected sample time-points were calculated and are shown in Table 2. The models showed similar predictive capabilities up to 24 h with the model derived without the 48-h time-point showing a range of MAPE% (25.2 to 68.9) and MPE% (5.6 to 68.9) similar to those seen with the model derived including the 48-h time-point (MAPE% 22.8 to 82.9 and MPE% −4.6 to 82.9). However, the model derived without the 48-h time-point was much worse at predicting the 48-h plasma levels (MAPE% 117.9) when compared to the model derived with the 48-h time-point (MAPE% 61.3). This is not surprising considering the difference in the gamma half-life between the two models and the fact that the model including these points is a better predictor. However, this does illustrate that the smaller gamma half-life associated with the model derived without the 48-h time-point can have a profound effect on its predictive ability.
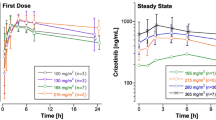
Pharmacokinetic parameters were also calculated with and without the 48-h time-point using non-compartmental modeling for docetaxel doses of 30 and 36 mg/m2 and the results are shown Tables 3 and 4, respectively. The results from the non-compartmental analysis were similar to those seen with compartmental modeling in that the AUC, CL and volume parameters were not significantly altered by the inclusion of the 48-h time-point (Figs 4 and 5). However, the estimated terminal half-life was significantly greater (P>0.05) when the 48-h time-point was included in the analysis. The effect of this when using these calculated parameters to predict docetaxel levels is illustrated in Fig. 6 and Table 5. When the Vext and the terminal elimination rate constant (λ) were used to predict the plasma levels during the linear, terminal phase of drug disposition, the parameters calculated without the inclusion of the 48-h time-point dramatically under-predicted the plasma levels. These results again illustrate the importance of an accurate calculation of the terminal half-life for calculation and extrapolation of plasma levels at later time-points.
Docetaxel pharmacokinetic parameters (A steady-state volume of distribution, Vss; B clearance, CL; C terminal half-life. t1/2λ) calculated by non-compartmental analysis in patients receiving 30 or 36 mg/m2 doses with and without the 48-h sampling time-point. Values are the means±SD for each calculated parameter. *P<0.05, values calculated with vs values calculated without the 48-h time-point
Docetaxel plasma time course data fitted to a non-compartmental model. The non-compartmental parameters Vext and t1/2λ were used to calculate the y-intercept and slope of the line representing the terminal elimination phase for a given set of parameters. The solid line represents the terminal elimination phase simulation using constants calculated with the 48-h time-point. The dashed line represents the terminal elimination phase simulation using constants calculated without the 48-h time-point
Discussion
The accurate determination of pharmacokinetic parameters is an important component in the development of effective drug therapies. Pharmacokinetic studies in oncology are especially critical and should be carried out in conjunction with phase I and phase II trials to allow correlations between important indicators of drug exposure and the therapeutic efficacy and/or toxicity of a treatment regimen to be determined. The taxanes are an important class of antineoplastic agents with proven efficacy against a variety of solid tumor types. These agents have traditionally been used in protocols based on the maximum tolerated dose (MTD) where patients are treated with the doses just below those resulting in dose-limiting toxicity. It has been presumed that these high doses followed by a recovery period and other subsequent treatments is the most effective way to kill tumor cells and prevent the development of drug resistance. Pharmacokinetic studies carried out using these types of protocols benefit by the relatively high doses of drug given which result in sustained patient plasma levels allowing full time-course sampling and analysis.
More recently, lower more-frequent dosing protocols have been gaining favor as potentially superior to MTD dosing [8, 14]. These low-dose more-frequent protocols have been termed "metronomic" dosing [12], and weekly lower-dose treatment with the taxanes has shown effective antitumor activity with less toxicity [2, 5, 27]. A problem with these lower-dose protocols is that pharmacokinetic analyses become more difficult due to the lower plasma levels associated with the lower doses. In our analysis, two patient samples would have been below the limit of detection using an HPLC-based assay at 8 h after dosing and none of the samples were above the limit of detection of 10 ng/ml at 24 h. The lack of data at later time-points can lead to a large degree of discrepancy in the calculation of pharmacokinetic parameters associated with the terminal elimination phase of the drug.
Docetaxel plasma pharmacokinetics have been described using a three-compartment model, with disposition half-lives of 4.5 min (α), 38.3 min (β) and 12.2 h (γ) following short (1–2 h) infusions [4]. These values are consistent with those obtained when calculating docetaxel pharmacokinetic parameters using samples only out to 24 h. However, when the 48-h time-point was included in the modeling, the terminal half-life was estimated to be approximately 60% longer whether calculated by compartmental or non-compartmental methods. The accurate determination of this terminal half-life is an important component when calculating the time that plasma levels stay above a threshold dose for therapy, and as shown in Tables 2 and 5 plasma concentrations will be underestimated if the terminal half-life is estimated to be lower than it actually is. It has been estimated that to get an accurate determination of the terminal half-life, studies need to extend to three to five times the duration of the actual terminal half-life [19]. This suggests that we actually need to sample out to between 60 and 100 h after dosing to get an accurate estimate of the terminal half-life of docetaxel in humans. The LCMS assay that we have developed is potentially sensitive enough to accurately determine plasma levels of docetaxel at these times, and such studies are planned for the future.
In summary, calculation of the pharmacokinetics of docetaxel using later time-points was made possible with the use of a more sensitive LCMS-based assay. This assay allowed complete sampling out to 48 h after dosing when docetaxel was given at lower doses (30–36 mg/m2) in a weekly protocol. Further, when the 48-h time-point was included in the calculation of the pharmacokinetic parameters both by compartmental and non-compartmental methods, the terminal half-life was estimated to be approximately 60% longer. Underestimation of the terminal half-life can have a large effect on the prediction of plasma levels at later time-points and can lead to the underestimation of plasma levels. Estimations can be a very important factor in the elucidation of pharmacokinetic and pharmacodynamic relationships in taxane activity in that the time that plasma levels are above a given threshold level may be substantially underestimated without the inclusion of later sampling time-points. Since the ability of docetaxel to inhibit endothelial cell proliferation has been shown to occur at low nanomolar levels [31], these correlations may be an important component of the pharmacology of metronomic dosing with docetaxel. For example, based on the three-compartment model parameters calculated with and without the inclusion of the 48-h time-point, the time that docetaxel plasma levels are above 1 nM would be estimated to be approximately 68 h and 55 h, respectively, at a dose of 30 mg/m2.
References
Beer TM, Pierce WC, Lowe BA, Henner WD (2001) Phase II study of weekly docetaxel in symptomatic androgen-independent prostate cancer. Ann Oncol 12:1273
Burstein HJ, Manola J, Younger J, Parker LM, Bunnell CA, Scheib R, Matulonis UA, Garber JE, Clarke KD, Shulman LN, Winer EP (2000) Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol 18:1212
Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D'Incalci M, Zucchetti M, Marmiroli P, Tredici G (2000) Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology 21:389
Clarke SJ, Rivory LP (1999) Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 36:99
Copur MS, Ledakis P, Lynch J, Hauke R, Tarantolo S, Bolton M, Norvell M, Muhvic J, Hake L, Wendt J (2001) Weekly docetaxel and estramustine in patients with hormone-refractory prostate cancer. Semin Oncol 28:16
Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A, Christodoulou CH, Timotheadou E, Kalofonos CH, Kosmidis P, Adamou A, Papakostas P, Gogas H, Stathopoulos G, Razis E, Bafaloukos D, Skarlos D (2001) Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol 12:1545
Garg MB, Ackland SP (2000) Simple and sensitive high-performance liquid chromatography method for the determination of docetaxel in human plasma or urine. J Chromatogr B 748:383
Gasparini G (2001) Metronomic scheduling: the future of chemotherapy? Lancet Oncol 2:733
Gianni L, Kearns CM, Giani A, Capri G, Vigano L, Lacatelli A, Bonadonna G, Egorin MJ (1995) Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 13:180
Gustafsson LL, Ebling WF, Osaki E, Harapat S, Stanski DR, Shafer SL (1992) Plasma concentration clamping in the rat using a computer-controlled infusion pump. Pharm Res 9:800
Hainsworth JD, Burris HA, Erland JB, Thomas M, Greco FA (1998) Phase I trial of docetaxel administered by weekly infusion in patients with advanced refractory cancer. J Clin Oncol 16:2164
Hanahan D, Bergers G, Bergsland E (2000) Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest 105:1045
Huizing MT, Misser VH, Pieters RC, ten Bokkel Huinink WW, Veenhof CH, Vermorken JB, Pinedo HM, Beijnen JH (1995) Taxanes: a new class of antitumor agents. Cancer Invest 13:381
Kamen BA, Rubin E, Aisner J, Glatstein E (2000) High-time chemotherapy of high time for low dose. J Clin Oncol 18:2935
Kerns EH, Hill SE, Detlefsen DJ, Volk KJ, Long BH, Carboni J, Lee MS (1998) Cellular uptake profile of paclitaxel using liquid chromatography tandem mass spectrometry. Rapid Commun Mass Spectrom 12:620
Leca F, Marchiset-Leca D, Noble A, Antonetti M (1991) New data on the pharmacokinetics of adriamycin and its major metabolite, adriamycinol. Eur J Drug Metab Pharmacokinet 16:107
Loesch D, Robert N, Asmar L, Gregurich MA, O'Rourke M, Dakhil S, Cox E (2002) Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol 20:3857
Pectasides D, Glotsos J, Bountouroglou N, Kouloubinis A, Mitakidis N, Karvounis N, Ziras N, Athanassiou A (2002) Weekly chemotherapy with docetaxel, gemcitabine and cisplatin in advanced transitional cell urothelial cancer: a phase II trial. Ann Oncol 13:243
Riviere JE (1999) Study design and data analysis. In: Riviere JE (ed) Comparative pharmacokinetics: principles, techniques and applications. Iowa State University Press, Ames, p 239
Rosing H, Lustig V, Koopman FP, ten Bokkel Huinink WW, Beijnen JH (1997) Bio-analysis of docetaxel and hydroxylated metabolites in human plasma by high-performance liquid chromatography and automated solid-phase extraction. J Chromatogr B 696:89
Schellen A, Ooms B, van Gils M, Halmingh O, van der Vlis E, van de Lagemaat D, Verheij E (2000) High throughput on-line solid phase extraction/tandem mass spectrometric determination of paclitaxel in human serum. Rapid Commun Mass Spectrom 14:230
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtube assembly in vitro by taxol. Nature 277:665
Schwonzen M, Kurbacher CM, Mallmann P (2000) Liposomal doxorubicin and weekly paclitaxel in the treatment of metastatic breast cancer. Anticancer Drugs 11:681
Seidman AD, Hudis CA, Albanel J, Tong W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L (1998) Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 16:3353
Sekine I, Nishiwaki Y, Watanabe K, Yoneda S, Saijo N (1996) Phase II study of 3-hour infusion of paclitaxel in previously untreated non-small cell lung cancer. Clin Cancer Res 2:941
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503
Socinski MA, Schell MJ, Bakri K, Peterman A, Lee J-H, Unger P, Yates S, Hudgens S, Kies MS (2002) Second-line, low-dose, weekly paclitaxel in patients with stage IIIB/IV nonsmall cell lung carcinoma who fail first-line chemotherapy with carboplatin plus paclitaxel. Cancer 95:1265
Sonnichsen DS, Relling MV (1998) Paclitaxel and docetaxel. In: Grochow LB, Ames MM (eds) A clinician's guide to chemotherapy pharmacokinetics and pharmacodynamics. Lippincott, Williams and Wilkins, Baltimore, p 375
Sonnichsen DS, Hurwitz CA, Pratt CB, Shuster JJ, Relling MV (1994) Saturable pharmacokinetics and paclitaxel pharmacodynamics in children with solid tumors. J Clin Oncol 12:532
Sottani C, Minoia C, D'Incalci M, Paganini M, Zucchetti M (1998) High-performance liquid chromatography tandem mass spectrometry procedure with automated solid phase extraction sample preparation for the quantitative determination of paclitaxel (Taxol) in human plasma. Rapid Commun Mass Spectrom 12:251
Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW Jr (2001) The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res 61:3369
Vergniol JC, Bruno R, Montay G, Frydman A (1992) Determination of taxotere in human plasma by a semi-automated high-performance liquid chromatographic method. J Chromatogr A 582:273
Wagner JG (1993) Noncompartmental and system analysis. In: Pharmacokinetics for the pharmaceutical scientist. Technomic Publishing Company, Lancaster, PA, p 83
Acknowledgements
This work was supported by CA75955 from the NCI to D.L.G. and by the University of Colorado Cancer Center Core grant (principle investigator: Dr. Paul A. Bunn, Jr.). The phase I clinical trial of docetaxel and exisulind in combination was supported by Aventis (Bridgewater, N.J.) and Cell Pathways (Horsham, Pa.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gustafson, D.L., Long, M.E., Zirrolli, J.A. et al. Analysis of docetaxel pharmacokinetics in humans with the inclusion of later sampling time-points afforded by the use of a sensitive tandem LCMS assay. Cancer Chemother Pharmacol 52, 159–166 (2003). https://doi.org/10.1007/s00280-003-0622-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0622-z