Abstract
The use of hypomethylating agents (HMAs) prior to hematopoietic stem cell transplantation (HSCT) in patients with myelodysplastic syndromes (MDS) was still controversial. Therefore, we sought to evaluate the impact of hypomethylation therapy before HSCT, with a special focus on long-term outcome. Databases, including PubMed, Embase Ovid, and the Cochrane Library, were searched for studies published up to 4 November 2018. Overall survival (OS) was selected as the primary endpoint, and relapse-free survival (RFS) was the secondary endpoint. A total of 6 cohort studies were included in the final meta-analysis. Our results showed that the outcome of patients with MDS using HMAs prior to HSCT was similar compared to those who did not with OS (HR = 0.81, 95% CI 0.63–1.04, p = 0.104) and RFS (HR = 0.96, 95% CI 0.72–1.26, p = 0.749). The pooled HR of OS in the older patients was 0.75 (95% CI 0.57–0.98, p = 0.035). No evidence showed that patients with MDS will benefit from using HMAs before HSCT in long-term survival (OS and RFS) compared to chemotherapy or best supportive therapy, though older patients were more likely to benefit from pre-transplantation HMAs treatment in terms of long-term survival. Our conclusions await further validation by prospective studies with larger sample size and randomized-controlled design. Particularly, to clarify whether the older patients who are candidates for HSCT could benefit from this bridging treatment will be of great interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelodysplastic syndrome (MDS) is a clonal hematopoietic disease characterized by ineffective hematopoiesis, cytopenia, and a tendency to transform to acute myeloid leukemia. MDS is also a group of diseases with such a high heterogenicity that its clinical manifestation can range from moderate single lineage cytopenia (i.e., anemia, thrombocytopenia, or leukopenia), to transfusion dependence, pancytopenia, and even rapid progression to acute myeloid leukemia. Over the past decade, DNA sequencing established that MDS arise through the sequential acquisition of somatic mutations in a set of recurrently involved genes. These driver genes can be divided into several categories, including RNA splicing factors, epigenetic regulators, cohesin components, transcription factors, the DNA damage response, signal transduction molecules, and the P53 pathway [1]. It has been proved that mutations of epigenetic modulation genes, such as DNMT3, TET2, and IDH1/2, contribute to both local and genome-wide hypermethylation of DNA, which underlie the evolution of ineffective hematopoiesis and clonal hematopoiesis. Hypomethylating agents (HMAs), including azacytidine (AZA) and decitabine (DAC), inhibit the activity of DNA methyltransferases (DNMTs) and reduce DNA hypermethylation. Additionally, AZA can also insert into RNA to interfere with the metabolism of malignant clones. Hypomethylation therapy is a standard treatment for MDS in the intermediate- and high-risk groups. The complete remission (CR) rate (with or without complete recovery of peripheral-blood counts) after HMA induction therapy typically ranged from 20 to 35% [2].
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is currently the only cure for MDS, with an overall survival (OS) rate ranging from 30 to 52%, a disease-free survival (DFS) rate ranging from 16 to 50%, and a treatment-related mortality (TRM) rate at 3 years after allogeneic HSCT ranging from 10 to 50% [3,4,5,6,7,8]. Two studies demonstrated the feasibility of administering decitabine prior to allo-HSCT in 2009 [9, 10]. In addition, there were studies showing that hypomethylation treatment improved patients’ prognoses [11,12,13,14,15,16,17]. However, the role of hypomethylation therapy before HSCT has also been questioned. Some believe that hypomethylation treatment might merely screen out patients with lower risk and better response to proceed with HSCT, since patients who failed the hypomethylation therapy often had more risk factors, such as TP53 mutations and complex chromosomal abnormalities, combined with myelofibrosis and older age. Whether patients with MDS would benefit from hypomethylation therapy before transplantation has remained controversial. Therefore, this study aimed at evaluating the impact of hypomethylation therapy before HSCT, focusing on long-term outcomes of patients by comprehensively collecting clinical studies on hypomethylation therapy in such clinical settings.
Materials and methods
Data sources and searches
We performed a comprehensive search using several databases: PubMed, Embase Ovid, and the Cochrane Library (from database inception through November 4, 2018). Our comprehensive search strategy is seen in the supplement (Supplementary Table 1A, Table 1B and Table 1C). We identified articles eligible for further review by screening abstracts and titles. Full texts of articles were obtained and reviewed for relevant studies. The protocol for this meta-analysis is available in PROSPERO (CRD42018116052).
Inclusion and exclusion
We included only those studies that met the following criteria: (1) the study focused on the prognostic impact of administering HMAs to MDS patients before HSCT; (2) the study provided sufficient survival data, at least on overall survival (OS); (3) the hazard ratio (HR) and its 95% confidence interval (95% CI) were directly reported or could be calculated from original data; (4) the study was published as a full-text article in English; (5) the study included human subjects; and (6) the article was not a review, case report, or animal study. If the same or overlapping data were presented in multiple studies, only the most recent or the highest-quality study was included. Disagreements were resolved by discussion.
Data extraction and outcome measures
Two reviewers independently extracted relevant information from each eligible study and input it into a spreadsheet. The data included the first author’s name, year of publication, country of origin, inclusion period, number of patients, age, HMA category, MDS subtype, criteria for classification of MDS, bone marrow blast count, and distribution of the International Prognostic Scoring System (IPSS) score, human leukocyte antigen (HLA) match status, stem cell source, and conditioning regimen. We selected overall survival (OS) as the primary endpoint and relapse-free survival (RFS) as the secondary endpoint. OS was defined as the time between transplantation and death or last follow-up for patients alive. RFS was defined as time interval between transplantation and first relapse or death without relapse, or last follow-up for patients alive in CR. Non-relapse mortality (NRM) was defined as death resulting from the transplantation procedure but without evidence of relapse. When HR was not reported, we tried to contact the author to obtain it or used the method reported before to calculate it [18].
Quality assessment
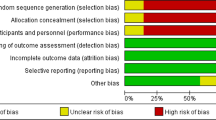
The methodological quality of each included study was independently evaluated by two reviewers. We assessed the quality of cohort studies by applying the Newcastle-Ottawa scale (NOS). The NOS sums up to nine points, including selection (four points), comparability (two points), and exposure or outcome (three points) [19]. Studies that scored six or more points were regarded as high quality [19] and eligible for our study. Divergences were resolved by discussion.
Statistical analysis
Calculations were carried out in Stata version 12.0 software (Stata Corp, College Station, TX, USA). The effects of administering HMAs on OS, RFS, and NRM were evaluated by calculating the combined HRs and their 95% CIs with the generic inverse variance method. A P value less than 0.05 was considered to be statistically significant. The chi-squared test was used to evaluate the heterogeneity of the studies, with the P value of less than 0.10 being significant. We used I2 statistic to quantify heterogeneity. For an I2 value of less than 25%, between 25 and 50%, and greater than 50%, the heterogeneity was considered to be low, moderate, and high, respectively. If a high degree of heterogeneity was detected, the random effect model was used; otherwise, the meta-analysis was performed using the fixed effect model. We also analyzed the source of heterogeneity by subgroup analysis. Sensitivity analysis was used to assess the stability of the pooled results by sequential omission of one study at a time. Publication bias was evaluated using funnel plots, Begg’s test, and Egger’s test [20].
Results
Search results
As shown in Fig. 1 (flow diagram of study selection), 3872 studies were searched from the databases. Three articles were added to the analysis by reference search. After excluding 1158 duplicates, 2717 citations were reviewed by screening the titles and abstracts; of those, 2626 citations were then excluded for irrelevant subjects or irrelevant study types. A total of 91 studies were left for full text review. Among these, 18 studies were excluded as meeting abstracts, and 74 studies were further excluded because of insufficient data or irrelevant outcomes. Six studies [21,22,23,24,25,26] were eventually included in the meta-analysis (see Fig. 1) (flow diagram of study selection).
Characteristics of included studies
The characteristics of the included studies are listed in Table 1. All these studies were retrospective cohort studies and were published between 2010 and 2016. Two studies were based in the USA, and one each was based in France, Japan, Korea, and Europe. All studies reported the effect of HMAs on OS. Five studies described RFS. Three studies reported the effect of HMAs on NRM. NOS score details are shown in Supplementary Table 2.
Clinical characteristics of patients
The total number of patients was 635, among which 278 were administered HMAs before HSCT. In all the included studies, except for one study in which patients received either AZA or DAC, AZA was the only HMA administered before HSCT. The IPSS score of the HMA group was also higher than that of the control group. Treatments in the control group included chemotherapy (both intensive chemotherapy (IC) [22] and conventional chemotherapy (CC) [26]) and the best supportive care (BSC) [24, 25]. Treatments in the other two control groups were any treatments other than HMA treatments [21, 23]. Six studies were retrospective studies. The rationale for treatment decision was not clearly stated in most of the articles included in this study. The only exception was Kim et al. [23], where the decision was based on clinical conditions and physician’s judgment. However, according to the comparison of patient characteristics in six studies, five of those studies showed that the age of the 5-AZA group was higher than that of the control group (P < 0.05). In the case of patients unsuitable for other chemotherapy and supportive treatment, the 5-AZA treatment may be safer, which could gain valuable time for pre-transplant preparations (seeking donors and economic support, etc.).
Analysis of outcomes
As shown in Fig. 2 (forest plots of pooled HR and 95% CIs for OS assessing the use of HMAs prior to HSCT), all cohort studies compared the effects of administering HMAs before HSCT on OS in patients with MDS. Our results showed that administration of HMAs before HSCT did not improve the OS (HR = 0.81, 95% CI, 0.63–1.04, P = 0.104), with a low heterogeneity among studies (I2 = 0%, P = 0.610). Five studies reported RFS data on 526 patients, among which 197 patients received HMA treatment before HSCT. The combined HR of RFS was 0.96 (95% CI, 0.72–1.26, P = 0.749), with a moderate heterogeneity among studies (I2 = 0%, P = 0.883) in patients in the HMA group compared with the control group (Supplementary Fig. 1), indicating that there was no improvement in RFS after administering HMAs before HSCT in MDS patients. The pooled HR for NRM was 0.79 (95% CI, 0.50–1.36, P = 0.452), with no heterogeneity among studies ( I2 = 0%, P = 0.524), showing no additional risk of mortality for administration of HMAs before HSCT (Supplementary Fig. 2).
Patients in the HMA group were older than the HMA-free patients in five studies (P < 0.05). The pooled HR of OS was 0.75 (95% CI, 0.57–0.98, P = 0.035, I2 = 0%), indicating that the older patients were more likely to benefit from pre-transplantation HMA treatment in terms of long-term survival (Fig. 3) (forest plots of pooled HR and 95% CIs for OS assessing the use of HMAs prior to HSCT in older patients). However, the pooled HR of RFS in the older patients was 0.90 (95% CI, 0.67–1.22, P = 0.509, I2 = 0%), showing no benefit from pre-transplantation HMA treatment (Supplementary Fig. 3).
Sensitivity analysis and publication bias
The sensitivity analysis was done by removing each study on pooled HRs of OS and RFS. The results showed that individual studies had no significant effect on the combined HR of OS (Supplementary Fig. 4A) or RFS (Supplementary Fig. 4B). In the funnel plots of OS (Supplementary Fig. 5A) and RFS (Supplementary Fig. 5A), the included studies showed no significant publication bias. The Begg’s test and Egger’s test showed no publication bias on OS (P = 0.060 for the Begg’s test and P = 0.177 for the Egger’s test) or RFS (P = 0.806 for the Begg’s test and P = 0.575 for the Egger’s test) either.
Discussion
In this study, we systematically analyzed all the currently available clinical studies on administering HMAs prior to allo-HSCT. Our results showed that patients with MDS who subsequently received HSCT did not further benefit from bridging treatment with HMAs. The major prognostic indicators (OS and RFS) after transplantation were not significantly improved in these patients compared to the HMA-naive patients (those who received BSC, CC, or IC instead). In terms of safety profile, the incidence of NRM in the HMA group was similar to that of the control group.
Patients aged from 60 to 75 constituted the majority of MDS patients. In a subgroup analysis, we found that for the older patient populations (≥ 60 years), administration of HMAs as a bridging treatment prior to HSCT could prolong patients’ OS, but not RFS. Allogeneic HSCT remained the only option for a cure. Because of significant bone marrow suppression and the unreasonably high risk of early death, chemotherapy apparently was not the best bridging treatment for this group of patients. Even though MDS could not be cured by HMA treatment, the advent of hypomethylation treatment allowed patients to achieve a better response, as evidenced by stable blood counts, blood transfusion independence, and an acceptable safety profile [11, 27, 28]. In this sense, hypomethylation treatment was conducive to HSCT, in that it could gain valuable time for pre-transplant preparations (seeking donors and economic support, etc.). The transplant mode of the reduce-intensity pre-treatment regimen (e.g., HMAs) might be more suitable for the aged patients. Therefore, it was necessary to control the disease by administering HMAs as a bridging treatment until a suitable stem cell donor was found and comorbidities were well controlled in most cases.
TP53 mutations and complex karyotype were more common in the older patients. TP53 mutations and/or complex karyotypes with multiple autosomal monomers had a high rate of poor response to chemotherapy alone for these patients [29,30,31,32,33]. However, several studies have shown that patients with MDS/AML with TP53 mutations and/or complex karyotypes had a good initial response rate to HMAs [17, 34, 35]. This suggests that administering HMAs prior to allo-HSCT might be particularly effective in patients with MDS or AML with complex karyotypes and/or TP53 mutations.
Disease status prior to transplantation remains a big concern for clinicians. Among the six studies, one [26] analyzed OS and RFS in patients with CR and those with primary refractory disease and found that the HR for OS was 2.93 (95% CI, 1.63–5.27; P < .001), while the HR for RFS was 2.56 (95% CI, 1.48–4.45; P = 001). However, two studies [23, 25] that compared AZA responders with non-responders found no significant difference between OS and RFS. One study [24] showed no significant difference in OS and RFS between respondents and non-responders in all HSCT patients. The incomplete data of all six studies made a comparison via data pooling impossible here. However, a more recent prospective study showed benefits in administering AZA prior to transplant if the patients achieved a CR [36]. Moreover, some studies also showed benefits of administering chemotherapy prior to transplant if the patients achieved a CR [3, 37]. According to the study of Voso et al. [36], 54 patients (56% of all 97 patients) received an allogeneic HSCT after a median of five cycles of AZA. Among them, 24 of the 54 patients (44%) were in CR at the time of HSCT. But in our paper, since all six studies focused on patients who received HSCT, it is unclear how many patients failed to undergo transplantation due to disease progression or HMAs/chemotherapy treatment-related mortality.
This study also had some limitations. First, outcomes obtained from some of the eligible studies were calculated by univariate analysis only, while the others were analyzed by the multivariate Cox proportional hazard model; this might account for the methodological heterogeneity across the six studies. Second, the number of participants included in the eligible studies was small, which made performing some subgroup analysis impossible. Again, there were many clinical heterogeneities among the studies, such as the types of HMAs (AZA versus DAC), the treatments applied in the control group (from BSC to CC or IC), age and gender distributions of patients, MDS subtypes, HLA disparities, and differences in conditioning regimens and lengths of follow-up, any of which might have influenced the clinical outcomes.
In summary, our meta-analysis showed that administering HMAs as a bridging therapy in patients with MDS prior to HSCT did not jeopardize the curative effect of HSCT as far as the long-term outcomes (i.e., OS, RFS, and NRM) were concerned. For the older patients who underwent HSCT, HMA bridging treatment appeared to improve their long-term survival rates. However, due to the limitations of the original studies, our conclusions await further validation by prospective studies with larger sample sizes and randomized-controlled designs. In particular, clarifying whether older patients who are candidates for HSCT would benefit from this bridging treatment would be of great interest.
References
Kennedy JA, Ebert BL (2017) Clinical implications of genetic mutations in myelodysplastic syndrome. J Clin Oncol 35(9):968–974. https://doi.org/10.1200/jco.2016.71.0806
Kim TK, Gore SD, Zeidan AM (2015) Epigenetic therapy in acute myeloid leukemia: current and future directions. Semin Hematol 52(3):172–183. https://doi.org/10.1053/j.seminhematol.2015.04.003
de Witte T, Hermans J, Vossen J, Bacigalupo A, Meloni G, Jacobsen N, Ruutu T, Ljungman P, Gratwohl A, Runde V, Niederwieser D, van Biezen A, Devergie A, Cornelissen J, Jouet JP, Arnold R, Apperley J (2000) Haematopoietic stem cell transplantation for patients with myelodysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol 110(3):620–630. https://doi.org/10.1046/j.1365-2141.2000.02200.x
Scott BL, Sandmaier BM, Storer B, Maris MB, Sorror ML, Maloney DG, Chauncey TR, Storb R, Deeg HJ (2006) Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia 20(1):128–135. https://doi.org/10.1038/sj.leu.2404010
Kindwall-Keller T, Isola LM (2009) The evolution of hematopoietic SCT in myelodysplastic syndrome. Bone Marrow Transplant 43(8):597–609. https://doi.org/10.1038/bmt.2009.28
Saber W, Cutler CS, Nakamura R, Zhang MJ, Atallah E, Rizzo JD, Maziarz RT, Cortes J, Kalaycio ME, Horowitz MM (2013) Impact of donor source on hematopoietic cell transplantation outcomes for patients with myelodysplastic syndromes (MDS). Blood 122(11):1974–1982. https://doi.org/10.1182/blood-2013-04-496778
Benjamin J, Chhabra S, Kohrt HE, Lavori P, Laport GG, Arai S, Johnston L, Miklos DB, Shizuru JA, Weng WK, Negrin RS, Lowsky R (2014) Total lymphoid irradiation-antithymocyte globulin conditioning and allogeneic transplantation for patients with myelodysplastic syndromes and myeloproliferative neoplasms. Biol Blood Marrow Transplant 20(6):837–843. https://doi.org/10.1016/j.bbmt.2014.02.023
Baron F, Zachee P, Maertens J, Kerre T, Ory A, Seidel L, Graux C, Lewalle P, Van Gelder M, Theunissen K, Willems E, Emonds MP, De Becker A, Beguin Y (2015) Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: a phase II randomized study from the Belgian Hematological Society. J Hematol Oncol 8. https://doi.org/10.1186/s13045-014-0098-9
Lubbert M, Bertz H, Ruter B, Marks R, Claus R, Wasch R, Finke J (2009) Non-intensive treatment with low-dose 5-aza-2 '-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone Marrow Transplant 44(9):585–588. https://doi.org/10.1038/bmt.2009.64
Silva LD, de Lima M, Kantarjian H, Faderl S, Kebriaei P, Giralt S, Davisson J, Garcia-Manero G, Champlin R, Issa JP, Ravandi F (2009) Feasibility of allo-SCT after hypomethylating therapy with decitabine for myelodysplastic syndrome. Bone Marrow Transplant 43(11):839–843. https://doi.org/10.1038/bmt.2008.400
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR, Int Vidaza High-Risk MDSS (2009) Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 10(3):223–232. https://doi.org/10.1016/s1470-2045(09)70003-8
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR (2010) Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 28(4):562–569. https://doi.org/10.1200/jco.2009.23.8329
Seymour JF, Fenaux P, Silverman LR, Mufti GJ, Hellstrom-Lindberg E, Santini V, List AF, Gore SD, Backstrom J, McKenzie D, Beach CL (2010) Effects of azacitidine compared with conventional care regimens in elderly (> = 75 years) patients with higher-risk myelodysplastic syndromes. Crit Rev Oncol Hematol 76(3):218–227. https://doi.org/10.1016/j.critrevonc.2010.04.005
Itzykson R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Salanoubat C, Visanica S, Stamatoullas A, Isnard F, Marfaing-Koka A, de Botton S, Chelghoum Y, Taksin AL, Plantier I, Ame S, Boehrer S, Gardin C, Beach CL, Ades L, Fenaux P, Gfm (2011) Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 117(2):403–411. https://doi.org/10.1182/blood-2010-06-289280
Lubbert M, Suciu S, Baila L, Ruter BH, Platzbecker U, Giagounidis A, Selleslag D, Labar B, Germing U, Salih HR, Beeldens F, Muus P, Pfluger KH, Coens C, Hagemeijer A, Schaefer HE, Ganser A, Aul C, de Witte T, Wijermans PW (2011) Low-dose decitabine versus best supportive care in elderly patients with intermediate-or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the european organisation for research and treatment of cancer leukemia group and the german MDS study group. J Clin Oncol 29(15):1987–1996. https://doi.org/10.1200/jco.2010.30.9245
Itzykson R, Thepot S, Quesnel B, Dreyfus F, Recher C, Wattel E, Gardin C, Ades L, Fenaux P (2012) Long-term outcome of higher-risk MDS patients treated with azacitidine: an update of the GFM compassionate program cohort. Blood 119(25):6172–6173. https://doi.org/10.1182/blood-2012-04-422204
Welch JS, Petti AA, Miller CA, Fronick CC, O'Laughlin M, Fulton RS, Wilson RK, Baty JD, Duncavage EJ, Tandon B, Lee YS, Wartman LD, Uy GL, Ghobadi A, Tomasson MH, Pusic I, Romee R, Fehniger TA, Stockerl-Goldstein KE, Vij R, Oh ST, Abboud CN, Cashen AF, Schroeder MA, Jacoby MA, Heath SE, Luber K, Janke MR, Hantel A, Khan N, Sukhanova MJ, Knoebel RW, Stock W, Graubert TA, Walter MJ, Westervelt P, Link DC, DiPersio JF, Ley TJ (2016) TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med 375(21):2023–2036. https://doi.org/10.1056/NEJMoa1605949
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8. https://doi.org/10.1186/1745-6215-8-16
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Begg CB, Mazumdar M (1994) Operating characteristics of a bank correlation test for publication bias. Biometrics 50(4):1088–1101. https://doi.org/10.2307/2533446
Field T, Perkins J, Huang Y, Kharfan-Dabaja MA, Alsina M, Ayala E, Fernandez HF, Janssen W, Lancet J, Perez L, Sullivan D, List A, Anasetti C (2010) 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 45(2):255–260. https://doi.org/10.1038/bmt.2009.134
Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL (2012) Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant 18(8):1211–1218. https://doi.org/10.1016/j.bbmt.2012.01.009
Kim Y, Kim I-H, Kim HJ, Park S, Lee K-H, Kim SJ, Lee J-H, Kim D-Y, Yoon S-S, Kim Y-K, Jang JH, Park SY, Ahn J-S, Cheong CW, Lee J-H, Cheong J-W, Korean Soc Hematology A (2014) Multicenter study evaluating the impact of hypomethylating agents as bridging therapy to hematopoietic stem cell transplantation in myelodysplastic syndromes. Int J Hematol 99(5):635–643. https://doi.org/10.1007/s12185-014-1549-3
Damaj G, Mohty M, Robin M, Michallet M, Chevallier P, Beguin Y, Nguyen S, Bodes P, Blaise D, Maillard N, Rubio MT, Fegueux N, Cornillon J, Clavert A, Huynh A, Ades L, Thiebaut-Bertrand A, Hermine O, Vigouroux S, Fenaux P, Duhamel A, Yakoub-Agha I (2014) Upfront allogeneic stem cell transplantation after reduced-intensity/nonmyeloablative conditioning for patients with myelodysplastic syndrome: a study by the Societe Francaise de Greffe de Moelle et de therapie cellulaire. Biol Blood Marrow Transplant 20(9):1349–1355. https://doi.org/10.1016/j.bbmt.2014.05.010
Oshikawa G, Yoshioka K, Takahashi Y, Shingai N, Ikegawa S, Kobayashil T, Doki N, Kakihana K, Ohashi K, Sakamaki H (2015) Impact of prior azacitidine on the outcome of allogeneic hematopoietic transplantation for myelodysplastic syndrome. Pathol Oncol Res 21(4):1037–1043. https://doi.org/10.1007/s12253-015-9933-8
Potter VT, Iacobelli S, van Biezen A, Maertens J, Bourhis J-H, Passweg JR, Yakhoub-Agha I, Tabrizi R, Bay J-O, Chevallier P, Chalandon Y, Huynh A, Cahn JY, Ljungman P, Craddock C, Lenhoff S, Russell NH, Fegueux N, Socie G, Benedetto B, Meijer E, Mufti GJ, de Witte T, Robin M, Kroeger N (2016) Comparison of intensive chemotherapy and hypomethylating agents before allogeneic stem cell transplantation for advanced myelodysplastic syndromes: a study of the myelodysplastic syndrome subcommittee of the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 22(9):1615–1620. https://doi.org/10.1016/j.bbmt.2016.05.026
Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF (2002) Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 20(10):2429–2440. https://doi.org/10.1200/jco.2002.04.117
Kantarjian H, Issa JPJ, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R, Shen LL, Nimer SD, Leavitt R, Raza A, Saba H (2006) Decitabine improves patient outcomes in myelodysplastic syndromes - resuits of a Phase III randomized study. Cancer 106(8):1794–1803. https://doi.org/10.1002/cncr.21792
Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, Kundgen A, Lubbert M, Kunzmann R, Giagounidis AAN, Aul C, Trumper L, Krieger O, Stauder R, Muller TH, Wimazal F, Valent P, Fonatsch C, Steidl C (2007) New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood 110(13):4385–4395. https://doi.org/10.1182/blood-2007-03-082404
Bowen D, Groves MJ, Burnett AK, Patel Y, Allen C, Green C, Gale RE, Hills R, Linch DC, Med Res Council Adult Leukaemia W (2009) TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia 23(1):203–206. https://doi.org/10.1038/leu.2008.173
Ruckner FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, Habdank M, Kugler CM, Holzmann K, Gaidzik VI, Paschka P, Held G, von Lilienfeld-Toal M, Lubbert M, Frohling S, Zenz T, Krauter J, Schlegelberger B, Ganser A, Lichter P, Dohner K, Dohner H (2012) TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 119(9):2114–2121. https://doi.org/10.1182/blood-2011-08-375758
Kayser S, Zucknick M, Dohner K, Krauter J, Kohne CH, Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Rummel M, Germing U, Gotze K, Nachbaur D, Schlegelberger B, Gohring G, Spath D, Morlok C, Teleanu V, Ganser A, Dohner H, Schlenk RF, German-Austrian AMLSG (2012) Monosomal karyotype in adult acute myeloid leukemia: prognostic impact and outcome after different treatment strategies. Blood 119(2):551–558. https://doi.org/10.1182/blood-2011-07-367508
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SMM, Miyazaki Y, Pfeilstocker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D (2012) Revised international prognostic scoring system for myelodysplastic syndromes. Blood 120(12):2454–2465. https://doi.org/10.1182/blood-2012-03-420489
Lubbert M, Ruter BH, Claus R, Schmoor C, Schmid M, Germing U, Kuendgen A, Rethwisch V, Ganser A, Platzbecker U, Galm O, Brugger W, Heil G, Hackanson B, Deschler B, Dohner K, Hagemeijer A, Wijermans PW, Dohner H (2012) A multicenter phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica 97(3):393–401. https://doi.org/10.3324/haematol.2011.048231
Lubbert M, Suciu S, Hagemeijer A, Ruter B, Platzbecker U, Giagounidis A, Selleslag D, Labar B, Germing U, Salih HR, Muus P, Pfluger KH, Schaefer HE, Bogatyreva L, Aul C, de Witte T, Ganser A, Becker H, Huls G, van der Helm L, Vellenga E, Baron F, Marie JP, Wijermans PW, Grp EL, German MDSSG (2016) Decitabine improves progression-free survival in older high-risk MDS patients with multiple autosomal monosomies: results of a subgroup analysis of the randomized phase III study 06011 of the EORTC Leukemia Cooperative Group and German MDS Study Group. Ann Hematol 95(2):191–199. https://doi.org/10.1007/s00277-015-2547-0
Voso MT, Leone G, Piciocchi A, Fianchi L, Santarone S, Candoni A, Criscuolo M, Masciulli A, Cerqui E, Molteni A, Finelli C, Parma M, Poloni A, Carella AM, Spina F, Cortelezzi A, Salvi F, Alessandrino EP, Rambaldi A, Sica S (2017) Feasibility of allogeneic stem-cell transplantation after azacitidine bridge in higher-risk myelodysplastic syndromes and low blast count acute myeloid leukemia: results of the BMT-AZA prospective study. Ann Oncol 28(7):1547–1553. https://doi.org/10.1093/annonc/mdx154
Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, Boulad F, Young JW, Kernan NA, Perales MA, Small TN, Hsu K, Chiu M, Heller G, Collins NH, Jhanwar SC, van den Brink M, Nimer SD, O'Reilly RJ (2008) Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant 14(4):458–468. https://doi.org/10.1016/j.bbmt.2008.02.006
Acknowledgments
We sincerely thank Yu-Tian Xiao (Department of Urology, Shanghai Changhai Hospital, Shanghai 200433, China) for providing assistance on prospective protocol registration and statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, Y., Kuang, P., Zeng, Q. et al. Hypomethylating agents for patients with myelodysplastic syndromes prior to hematopoietic stem cell transplantation: a systematic review and meta-analysis. Ann Hematol 98, 2523–2531 (2019). https://doi.org/10.1007/s00277-019-03811-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03811-x