Abstract
Scarce information is available on the use of ponatinib as second-line treatment in chronic phase chronic myeloid leukemia (CP-CML) patients resistant and/or intolerant to prior tyrosine kinase inhibitor (TKI) therapy. We collected data from 29 CML patients, with a median age of 54 years (range 32–72). Eleven patients had received dasatinib, 15 patients received nilotinib, and 3 patients received imatinib as first-line treatment. Forty-five percent of patients started ponatinib for secondary resistance, 38% for primary resistance, 7% for severe intolerance associated to a molecular warning, 7% due to the presence of a T315I mutation, and 3% for severe intolerance. Ponatinib was started at a dose of 45 mg in 60% of patients, 30 mg in 38%, and 15 mg in 2% of patients. Overall, at a median follow-up of 12 months, 85% of treated patients improved the level of response as compared to baseline, with 10 patients achieving a deep molecular response (MR4-4.5). No thrombotic events were recorded. The dose was reduced during treatment in 2 patients due to intolerance and in 8 patients in order to reduce the cardiovascular risk. Ponatinib seems a valid second-line treatment option for chronic phase CML, in particular for patients who failed a front-line second-generation TKI due to BCR-ABL-independent mechanisms of resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ponatinib is a third-generation tyrosine kinase inhibitor (TKI) designed to overcome the gatekeeper T315I mutation. In different trials, this drug has shown inhibitory activity against the native BCR-ABL kinase and several ABL mutations [1, 2]. For this reason, ponatinib is currently indicated for the treatment of chronic myeloid leukemia (CML) patients in every phase of the disease resistant and/or intolerant to dasatinib and nilotinib, and for whom imatinib is no longer indicated or for patients carrying the T315I mutation [3]. The drug is also indicated for Ph+ acute lymphoblastic leukemia (ALL). Ponatinib was temporarily suspended in 2013 for the occurrence of cardiovascular thrombotic events. Since then, different investigators have analyzed the baseline characteristics of patients candidate for ponatinib, in particular the cardiovascular profile, in order to provide general management recommendations [4, 5]. In the open-label, multinational phase II ponatinib Ph+ ALL and CML Evaluation (PACE, NCT01207440) trial, the drug was tested in a large cohort of 449 patients resistant or intolerant to nilotinib or dasatinib, or carrying the T315I mutation: 270 chronic phase (CP) CML, 85 accelerated phase (AP) CML, 62 blast crisis (BP) CML, and 32 Ph+ ALL patients. At the last update, after a median follow-up of 37.3 months, 41% of CP CML patients were still on study. The cumulative response rates, evaluated in terms of major (MCyR) and complete cytogenetic response (CCyR), major molecular response (MMR), and MR4.5, were 59, 54, 39, and 23%, respectively [2]. In this trial, only 16 patients were treated after a previous resistance to one TKI, but the characteristics of this subset of patients have never been reported. Very limited information is available on ponatinib administered as second-line treatment after resistance and/or intolerance to a single line of therapy in CP-CML. The aim of this study was thus to collect data related to the use of ponatinib as second-line treatment in CP-CML patients outside of a clinical trial.
Patients and methods
We retrospectively collected data related to the use of ponatinib as second-line treatment in CP-CML patients after each available front-line TKI in order to report efficacy and safety outside of a clinical trial, with a particular attention to the incidence of cardiovascular events. Each participating center provided the required data, after obtaining an informed consent according to ethics committee. No patient was excluded and also patients who discontinued for toxicity have been considered. All patients were treated in label as suggested by ponatinib indications, except one treated off label. We only excluded patients in advanced phase of their disease (accelerated or blast phase). The endpoints of this study were to evaluate the efficacy of ponatinib at 3, 6, and 12 months and the last follow-up; evaluate the effective dose used and overall survival; and define the safety profile. Patients were monitored during ponatinib according to European LeukemiaNet recommendations: conventional cytogenetics was performed every 3 months until CCyR was achieved, and quantitative molecular analysis was performed every 3 months from peripheral blood.
Results
Overall, 29 patients have been collected: median age of 54 years (range 32–72); 17 were males and 12 were females. According to the Sokal risk at baseline, 2 patients were classified as low risk, 17 as intermediate risk, and 10 as high risk. According to the Eutos score, 18 patients were low risk and 11 were high risk. At baseline, 2 patients have major additional cytogenetic aberrations (ACA) on Ph+ cells and 1 patient has a minor ACA. Eleven patients received dasatinib as first-line treatment, 15 received nilotinib, and 3 patients were treated with imatinib. Median time from diagnosis to ponatinib treatment was 16 months. The best response achieved before to switch to ponatinib was only a complete hematologic response without cytogenetic response in 6 patients (20.6%), a minimum cytogenetic response in 3 patients (10.3%), a CCyR in 6 patients (20.6%), a MMR in 4 patients (10.3%), and none in 10 patients (34%). Thirteen patients (45%) started ponatinib for secondary resistance (only 5 patients due to the onset of mutations different from T315I, 1 patient after imatinib, 5 after dasatinib, 7 after nilotinib), 11 patients (38%) for primary resistance (9 patients for primary cytogenetic resistance and 2 patients for hematologic resistance, 6 patients after nilotinib and 5 after dasatinib treatment), 2 patients (7%) for severe intolerance associated to a molecular warning (2 patients after nilotinib), 2 patients (7%) due to the presence of the T315I mutation (after imatinib failure), and 1 patient for severe intolerance to dasatinib (Table 1). Other mutations were reported as cause of secondary resistance in 5 patients: F359V in 3 patients and Y253H and Q252H in 2 further patients.
Ponatinib was started at the dose of 45 mg in 59% of patients, 30 mg in 38% of patients, and 15 mg in 3% of patients. None of the patients had previous cardiovascular events, but 2 patients were affected by hypertension, 1 by diabetes, 1 patient by a dilatative cardiomyopathy, and 1 patient by hypercholesterolemia: the dose was reduced at baseline in some patients only for age and pre-existing cardiovascular risk factors. The dose was reduced during treatment in 2 patients (for intolerance from 45 to 30 mg) and in 8 patients in order to reduce the cardiovascular risk after obtaining a cytogenetic response (from 45 to 30 mg in 4 patients and from 30 to 15 mg in 4 patients). At a median follow-up of 12 months (range 3-24, only 5 patients with less than 6 months of treatment), 2 patients achieved a partial cytogenetic response (PCyR), 2 patients a CCyR, 11 patients a MMR and 10 patients a deep molecular response (MR4-4.5, including 2 patients with T315I) as best response (Table 2). Four patients did not obtain a response. We did not find differences in the degree of response in patients carrying a mutation at baseline. In terms of cumulative incidence of response at 3 months, 78.5% of patients achieved an early molecular response (BCR-ABL1 level IS < 10%), 54% of patients achieved a MMR at 6 months, and 32% of patients achieved a MR4.5 at 12 months.
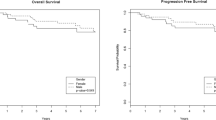
One patient progressed but none died during treatment (overall survival 100%, event-free survival 96%). Overall, 85.7% of treated patients improved the level of response as compared to baseline.
The most common side effects recorded were increased lipase (2 patients), hypertension (3 patients), skin rash (5 patients), and thrombocytopenia grade 3 (5 patients). Neutropenia grade 3 was recorded in 3 patients. None of the patients discontinued due to toxicity. We did not observe cardiovascular side effects.
Discussion
We previously reported very preliminary results in 10 CML-CP patients treated with ponatinib as second-line treatment [6], confirming the activity of this drug in this clinical setting. In the present paper, we have updated this small series and have collected data from 19 further patients. Overall, only 3 patients were treated with imatinib and 2 of them experienced secondary resistance due to the onset of a T315I mutation. Except for 5 patients in which a mutation was revealed as the reason of the secondary resistance, in the series hereby described, all patients were treated with a second-generation TKI without a recognized cause of primary or secondary resistance. Very limited information is presently available on the use of ponatinib in the setting of patients resistant only to a first-line TKI treatment: in the PACE trial (NCT01207440) [2], responses were higher as compared to those in patients treated in third or subsequent line with a CCyR rate of 74% and a MMR rate of 47%. Responses were also higher in the report by Sanford et al. [7], which described 5 patients who received ponatinib as second-line treatment (3 after imatinib and 2 after dasatinib failure) included in a phase II trial that prematurely closed after FDA recommendations based on safety concerns. No cardiovascular safety concerns were observed in the present experience, confirming our preliminary results [6]. The possible reasons are in the limited follow-up, limited previous exposure to other TKIs, better selection of patients with few concomitant comorbidities, and lack of pre-existing cardiovascular predisposing risk factors. Also, the use of a low starting dose of the drug in 40% of patients and the subsequent adaptation to the full dose in 10 patients may have contributed to the absence of cardiovascular side effects. This is in line with what reported in the PACE trial in which the dose was reduced in patients who had achieved the primary endpoint with a reduction in thrombotic event rates [2]. A pharmacokinetic analysis of the trial showed that a dose reduction to 15 mg/day was associated with a 40% relative risk reduction of thrombotic events [8].
Two ongoing trials are indeed investigating the most appropriate starting dose of ponatinib for CML patients: the OPTIC study (NCT02467270), a phase II trial that compares a 15-, 30-, and 45-mg daily dose of ponatinib in CP-CML patients resistant to at least two prior TKIs, and the OPUS trial (NCT02398825) that enrolls patients resistant to imatinib as first-line treatment and who receive ponatinib at the dose of 30 mg.
In summary, our data confirm the role of ponatinib in the second-line setting, in particular in those patients with likely BCR-ABL1-independent mechanisms of resistance treated front line with a second-generation TKIs. Prospective analyses are warranted in larger cohort of patients to confirm the efficacy and the positive safety profile observed.
References
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DiPersio J, DeAngelo DJ, Abruzzese E, Rea D, Baccarani M, Müller MC, Gambacorti-Passerini C, Wong S, Lustgarten S, Rivera VM, Clackson T, Turner CD, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes T, Goldman JM, Shah NP, Kantarjian H, PACE Investigators (2013) PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. New Engl J Med 369:1783–1796
Cortes JE, Kim DW, Pinilla-Ibarz J, et al (2014) Long-term follow-up of ponatinib efficacy and safety in the phase 2 PACE trial. Blood 124: abstract 3135
Poch Martell M, Sibai H, Deotare U, Lipton JH (2016) Ponatinib in the therapy of chronic myeloid leukemia. Exp Rev Hematol 9:923–932
Breccia M, Pregno P, Spallarossa P, Arboscello E, Ciceri F, Giorgi M, Grossi A, Mallardo M, Nodari S, Ottolini S, Sala C, Tortorella G, Rosti G, Pane F, Minotti G, Baccarani M (2017) Identification, prevention and management of cardiovascular risk in chronic myeloid leukemia patients candidate to ponatinib: an expert opinion. Ann Hematol 96:549–558
Muller MC, Cervantes F, Hjorth-Hansen H, Janssen JJWM, Milojkovic D, Rea D, Rosti G (2017) Ponatinib in chronic myeloid leukemia (CML): consensus on patient treatment and management from a European expert panel. Crit Rev Oncol Hematol 120:52–59
Breccia M, Abruzzese E, Iurlo A, Gozzini A, Isidori A, Gangemi D, Pregno P, Alimena G (2016) Efficacy and safety of second-line ponatinib after failure of a single previous tyrosine kinase inhibitor for chronic myeloid leukemia patients in chronic phase. Haematologica 101:e267–e268
Sanford D, Kantarjian H, Skinner J, Jabbour E, Cortes J (2015) Phase II trial of ponatinib in patients with chronic myeloid leukemia resistant to one previous tyrosine kinase inhibitor. Haematologica 100(suppl 1):e495
Hochhaus A, Pinilla-Ibarz J, Kim DW, et al (2014) Clinical impact of dose modification and dose intensity on response to ponatinib (PON) in patients (pts) with Philadelphia chromosome-positive (Ph+) leukemias. J Clin Oncol 32(15, suppl): abstract 708
Author information
Authors and Affiliations
Contributions
MB designed the study, analyzed the data, and wrote the manuscript; EA, AG, AI, PP, AI, DG, MG, MBo, FC, FS, LL, MBoc, DL, AM, NG, MC, IC, MP, and ARS collect data; RF critically revised the paper and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
MB received honoraria for speaking by Incyte, Pfizer, Novartis, and BMS.
Rights and permissions
About this article
Cite this article
Breccia, M., Abruzzese, E., Castagnetti, F. et al. Ponatinib as second-line treatment in chronic phase chronic myeloid leukemia patients in real-life practice. Ann Hematol 97, 1577–1580 (2018). https://doi.org/10.1007/s00277-018-3337-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3337-2