Abstract
Pneumonitis is a rare but severe and potentially fatal adverse effect in chemotherapy of lymphoma. This study is aimed to investigate the incidence of interstitial pneumonitis in non-Hodgkin’s lymphoma (NHL) patients receiving immunochemotherapy with pegylated liposomal doxorubicin and rituximab. Lymphoma patients were retrospectively reviewed, and eligible patients were included in this study. According to the chemotherapy regimens, patients were classified in four groups: combination of vincristine, cyclophosphamide, doxorubicin, and prednisone (CHOP group) with rituximab (RCHOP group) and combination of vincristine, cyclophosphamide, pegylated liposomal doxorubicin and prednisone (CDOP group) with rituximab (RCDOP group). Incidence and severity of interstitial pneumonitis were compared among the four groups. Among 757 patients reviewed, 207 patients were included in final analysis. Thirteen patients developed chemotherapy-induced interstitial pneumonitis, and the mean cycle of chemotherapy before the onset of pneumonitis was 4. Incidence rates of pneumonitis were 0, 1.8, 17.4, and 21.1% in CHOP, RCHOP, CDOP, and RCDOP groups, respectively (p < 0.001). The mean grades of pneumonitis were 0, 2, 2.5, and 3 in four groups, respectively (p < 0.001). After adjustment of confounders, chemotherapy regimens (OR 3.491, 95% CI 1.527–7.981, p = 0.003) and neutropenia in previous cycles (OR 2.186, 95% CI 1.281–3.731, p = 0.004) were independently associated with the incidence of pneumonitis. Interstitial pneumonitis should be highlighted in NHL patients who received more than 4 cycles of RCDOP chemotherapy regimen, especially in those who had grade 4 neutropenia in the previous cycles of chemotherapy.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Non-Hodgkin’s lymphoma (NHL) is one of the most common tumors in the world [1, 2], and its incidence has significantly increased in recent decades [3]. It comprises a group of diverse subtypes, such as diffuse large B cell lymphoma (DLBCL), Follicular lymphoma (FL), mucosa-associated lymphoid tissue lymphoma (MALT), mantle cell lymphoma (MCL), and peripheral T cell lymphoma (PTCL) [4, 5]. Among the diverse subtypes of NHL, DLBCL is the most frequent subtype and represents more than 30% of NHL in adults [6]. Ever since the anti-CD20 monoclonal antibody rituximab was developed and used clinically, the prognosis of NHL was dramatically improved [7,8,9]. At present, the combination of vincristine, cyclophosphamide, doxorubicin, and prednisone (CHOP) with rituximab (R) is the standard treatment for non-Hodgkin’s lymphoma [10].
However, NHL patients receiving RCHOP regimen always suffer several treatment-related adverse effects including hematologic toxicities, infections, alopecia, or cardiologic complications [11, 12], and doxorubicin is the agent with most frequent side effects in the RCHOP regimen, especially cardiac toxicity [13, 14]. A new formulation of doxorubicin, called pegylated liposomal doxorubicin, has extended circulation time and reduced volume of distribution than doxorubicin [15]. Previous studies suggested that the cardiotoxicity of pegylated liposomal doxorubicin was less than doxorubicin [16,17,18,19], and the combination of vincristine, cyclophosphamide, pegylated liposomal doxorubicin, and prednisone (CDOP) with rituximab (R) was an alternative regimen for non-Hodgkin’s lymphoma used in clinical practice [18, 20, 21].
Apart from cardiotoxicity, pneumonitis is also a severe and potentially fatal adverse effect in chemotherapy of lymphoma. It was reported that the addition of rituximab to CHOP regimen increased the incidence of interstitial pneumonitis in patients with NHL [22,23,24,25]. In addition, several case reports [26,27,28] or small sample studies [20, 29,30,31,32] highlighted that interstitial pneumonitis was a more frequent and potentially fatal side effect of pegylated liposomal doxorubicin.
To date, there is still lack of studies focused on incidence of pneumonitis in NHL patients receiving immunochemotherapy of RCDOP, and whether the combination of rituximab and pegylated liposomal doxorubicin increase the incidence of pneumonitis is still unclear. Therefore, the aim of this study is to investigate the incidence and severity of treatment-related interstitial pneumonitis among patients receiving chemotherapy of CHOP, RCHOP, CDOP, and RCDOP and also to identify variables associated with incidence of pneumonitis.
Methods
Patients and data collection
Lymphoma patients between December 2014 to June 2017 who were treated in Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology were retrospectively reviewed. Patients who received RCHOP, CHOP, RCDOP, or CDOP were included in this study. Patients who had uncertain diagnosis or received less than 2 cycles of chemotherapy were excluded. This study was reviewed and approved by the Tongji Medical College Research Ethics Board.
Patients’ baseline demographic and clinical characteristics including gender, age, lymphoma types, and Ann Arbor stages were collected from medical records. Chemotherapy-related information including chemotherapy regimen and cycles, chemotherapy-induced neutropenia, and chemotherapy-induced symptoms of pneumonitis (e.g., cough, fever, chest congestion, or dyspnea) were reviewed during the whole treatment. Moreover, information of chest computed tomography (CT) scans performed on patients with clinical indications of pneumonitis were recorded.
Chemotherapy-induced interstitial pneumonitis
Patients with chest CT scans showed interstitial pulmonary infection and any concomitant symptoms of pneumonitis including fever, cough, chest congestion, or dyspnea were considered as symptomatic pneumonitis. Chemotherapy-induced pneumonitis was defined as symptomatic pneumonitis which occurred within 60 days of the most recent chemotherapy cycle that could not be explained by other factors, such as lymphoma pulmonary invasion or radiation-induced pneumonitis. Incidence and severity of symptomatic pneumonitis were investigated in this study.
Statistical analysis
Patients’ demographic and clinical characteristics were summarized by descriptive statistics. Category variables were compared among NHL patients receiving CHOP, RCHOP, CDOP, and RCDOP regimens through chi-square test, but when more than one-fifth expected frequencies < 5 or one expected frequency < 1, Fisher’s exact test was used instead. For continuous variables, one-way ANOVA was used for comparison of the four groups in variables with variance homogeneity; otherwise, Kruskal-Wallis Test was used instead. In order to adjust for confounders of baseline demographic and clinical characteristics, such as gender, age, Eastern Cooperative Oncology Group (ECOG), lymphoma types, stages, international prognostic index (IPI), and chemotherapy cycles, multivariate logistic regression was applied for analysis of factors associated with incidence of pneumonitis. SPSS version 18.0 was used for statistical analyses and p value < 0.05 was defined as statistically significant.
Results
A total of 757 patients with lymphoma were reviewed. Among them, 389 patients who did not receive CHOP, RCHOP, CDOP, or RCDOP as their chemotherapy regimen and 161 patients who received less than 2 cycles of chemotherapy were excluded. Finally, 207 patients with non-Hodgkin’s lymphoma were included for analysis.
According to chemotherapy regimen, 207 patients were classified in four groups: CHOP group (n = 89), RCHOP group (n = 57), CDOP group (n = 23), and RCDOP group (n = 38). Patients’ demographic and clinical characteristics were summarized in Table 1. The mean ages of the four groups were 52, 46, 49, and 51 years, respectively (p = 0.070). Female patients were more than male patients in CHOP group (55.1%) and less than male patients in RCHOP (35.1%), CDOP (21.7%), and RCDOP (42.1%) groups (p = 0.012). None of patients had Eastern Cooperative Oncology Group (ECOG) more than 3, and the majority of patients had ECOG 0–1 (p = 0.252). DLBCL was the most common diagnosis in our patients, and other common existing subtypes of lymphoma included FL, MALT, and MCL (p = 0.065). About half of the patients were in advanced stage (p = 0.581), and approximately 40% of patients were in low risk with international prognostic index (IPI) 0–1(p = 0.306). The mean cycles of chemotherapy were 5, 5, 3, and 5 in the four groups (p < 0.001), respectively.
Among 207 patients, symptomatic interstitial pneumonitis occurred in 13 patients, and details of these patients were listed in Table 2. The median age of these patients was 49 years with a range of 20 to 72 years, and more than three quarters of patients were male patients. The most common subtype of lymphoma was DLBCL in our patients, and more than 60% of patients were in advanced stage (stage III/IV). The mean cycle of chemotherapy before pneumonitis was 4 in these patients, and they had a median grade 4 of neutropenia during chemotherapy cycles.
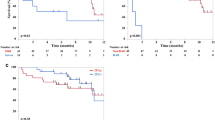
As shown in Fig. 1, 21.1, 17.4, and 1.8% of patients suffered symptomatic interstitial pneumonitis in RCDOP, CDOP, and RCHOP groups, respectively, and no patients experienced pneumonitis in CHOP group. Incidence rates of pneumonitis in RCDOP and CDOP groups were apparently higher than RCHOP and CHOP groups, and the difference was statistically significant (p < 0.001). The mean grades of pneumonitis were 0, 2, 2.5, and 3 in patients with pneumonitis in CHOP, RCHOP, CDOP, and RCDOP groups, respectively (Fig. 2). Interstitial pneumonitis was severe in RCDOP group than in CDOP group or RCHOP group, and the difference was statistically significant among the four groups (p < 0.001). Among the 13 patients with pneumonitis, chemotherapy was not interrupted in four patients with grade 2 pneumonitis; in other two patients with grade 2 pneumonitis and two patients with grade 3 pneumonitis, rituximab was given up in the following chemotherapy cycles; chemotherapy was discontinued in other two patients with grade 3 pneumonitis and three patients with grade 4 pneumonitis.
As summarized in Table 3, chemotherapy regimens (OR 3.491, 95% CI 1.527–7.981, p = 0.003) and neutropenia in previous cycles (OR 2.186, 95% CI 1.281–3.731, p = 0.004) were independently associated with the incidence of pneumonitis, while patients’ gender, age, ECOG, lymphoma types, stages, IPI, and chemotherapy cycles were not associated with incidence of pneumonitis (all p > 0.05).
Discussion
To our knowledge, this study is among very rare studies that focused on interstitial pneumonitis in NHL patients receiving immunochemotherapy with rituximab and pegylated liposomal doxorubicin. In this study, we found that NHL patients receiving RCDOP regimen experienced highest prevalence and most severity of interstitial pneumonitis. Moreover, chemotherapy regimens and neutropenia in previous cycles were independently associated with the incidence of pneumonitis.
Several previous studies reported the incidence of interstitial pneumonitis in NHL patients receiving immunochemotherapy with rituximab. In the study of Liu X et al. [33], 8.4% of NHL patients receiving rituximab-containing chemotherapy developed interstitial pneumonitis. In another study of Salmasi, G. et al. [34], incidence of interstitial pneumonitis was 3.95% in NHL patients receiving RCHOP or RCVP chemotherapy, and it was 1.3% in NHL patients receiving CHOP or CVP chemotherapy. In a study of interstitial pneumonitis in patients with diffuse large B cell lymphoma [35], interstitial pneumonitis was found in 4.9% of patients receiving first-line tri-weekly COP- or CHOP-based chemotherapy with or without rituximab. In another study of Katsuya, H. et al. [22], interstitial pneumonitis occurred in 1.7% of NHL patients receiving CHOP chemotherapy, and it occurred in 6.2% of NHL patients receiving RCHOP chemotherapy. In comparison with these studies, incidence of interstitial pneumonitis was lower in our patients with CHOP or RCHOP regimens, partly due to different populations and the different chemotherapy regimens included in these studies.
Interstitial pneumonitis of pegylated liposomal doxorubicin was reported in a few studies of patients with hematological malignant tumors. In a phase II study of pegylated liposomal doxorubicin combination chemotherapy for patients with multiple myeloma [29], the incidence of pneumonitis was 15%. In another study of pegylated liposomal doxorubicin combination chemotherapy in Hodgkin’s lymphoma patients [30], 5 of 30 patients experienced grades 3–5 pneumonitis. In comparison with these studies, incidence of interstitial pneumonitis of pegylated liposomal doxorubicin was higher in our results, and the reason could be the differences of chemotherapy regimens and diagnoses of patients in these studies.
Cardiotoxicity was always highlighted in studies of lymphoma patients receiving immunochemotherapy containing pegylated liposomal doxorubicin and rituximab [17,18,19, 36], but few studies reported the incidence of pneumonitis. In a phase II study of splenic marginal zone lymphoma [21], 51 patients received RCDOP immunochemotherapy; however, one patient died during treatment because of pneumonitis, which confirmed that pneumonitis was a severe and fatal adverse effect in patients receiving RCDOP regimen.
In our study, the mean cycle of chemotherapy before the onset of pneumonitis was four, which was consistent with the study of patients with DLBCL receiving rituximab-containing chemotherapy [35]. Apart from chemotherapy regimens, neutropenia was considered as another risk factor associated with the incidence of pneumonitis. Although no study has analyzed the association between neutropenia and pneumonitis in lymphoma patients, low lymphocyte count was reported as a risk factor for incidence of pneumonitis in previous studies [22, 35].
This study also had some limitations. Firstly, this study was a retrospective study and the respiratory symptoms of patients were reviewed from medical records, which may lead to information bias and influence the incidence and severity of interstitial pneumonitis in our patients. Secondly, a large number of patients were reviewed in our study, but many patients were excluded due to chemotherapy regimens other than CHOP/RCHOP/CDOP/RCDOP or use of less than 2 cycles of chemotherapy. The limited number of patients included in each group, especially the low number of patients and low number of chemotherapy cycles in CDOP group, may affect the results of our study. Finally, this study was conducted in a single center, and the generalizability of our results may be limited.
In conclusion, incidences of interstitial pneumonitis were higher in NHL patients receiving RCDOP and CDOP regimens than RCHOP and CHOP regimens, and interstitial pneumonitis was severe in NHL patients receiving RCDOP chemotherapy than CDOP chemotherapy. Replacement of doxorubicin by pegylated liposomal doxorubicin in NHL patients receiving CHOP regimen with or without rituximab could increase the incidence of interstitial pneumonitis. Moreover, addition of rituximab in chemotherapy regimen of CDOP could aggravate the severity of interstitial pneumonitis. The mean cycle of chemotherapy before the onset of pneumonitis was four. Apart from chemotherapy regimens, neutropenia was also a risk factor that was independently associated with the incidence of pneumonitis. Therefore, interstitial pneumonitis should be highlighted in NHL patients who received more than 4 cycles of immunochemotherapy containing pegylated liposomal doxorubicin and rituximab, especially in those who had grade 4 neutropenia in the previous cycles of chemotherapy. Further prospective studies with large samples are needed for confirming our results.
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. Ca-Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Chen WQ, Zheng RS, Baade PD, Zhang SW, Zeng HM, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China, 2015. Ca-Cancer J Clin 66(2):115–132. https://doi.org/10.3322/caac.21338
Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Byrd JC, Czuczman MS, Fayad L, Forero A, Glenn MJ, Gockerman JP, Gordon LI, Harris NL, Hoppe RT, Horwitz SM, Kaminski MS, Kim YH, Lacasce AS, Mughal TI, Nademanee A, Porcu P, Press O, Prosnitz L, Reddy N, Smith MR, Sokol L, Swinnen L, Vose JM, Wierda WG, Yahalom J, Yunus F (2010) NCCN clinical practice guidelines in oncology: non-Hodgkin's lymphomas. J Natl Compr Cancer Netw : JNCCN 8(3):288–334. https://doi.org/10.6004/jnccn.2010.0021
Horwitz SM, Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Byrd JC, Fayad LE, Fisher RI, Glenn MJ, Habermann TM, Lee Harris N, Hernandez-Ilizaliturri F, Hoppe RT, Kaminski MS, Kelsey CR, Kim YH, Krivacic S, LaCasce AS, Lunning M, Nademanee A, Press O, Rabinovitch R, Reddy N, Reid E, Roberts K, Saad AA, Sokol L, Swinnen LJ, Vose JM, Yahalom J, Zafar N, Dwyer M, Sundar H, Porcu P (2016) NCCN guidelines insights: non-Hodgkin's lymphomas, version 3.2016. J Natl Compr Cancer Netw : JNCCN 14(9):1067–1079. https://doi.org/10.6004/jnccn.2016.0117
Jiang ML, Bennani NN, Feldman AL (2017) Lymphoma classification update: B-cell non-Hodgkin lymphomas. Expert Rev Hematol 10(5):405–415. https://doi.org/10.1080/17474086.2017.1318053
Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS (2015) Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol 90(9):790–795. https://doi.org/10.1002/ajh.24086
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van den Neste E, Salles G, Gaulard P, Reyes F, Gisselbrecht C (2002) CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. New Engl J Med 346(4):235–242. https://doi.org/10.1056/Nejmoa011795
Cetiner M, Salepci T, Atesoglu EB, Gumus M, Guven A, Undar L, Tuglular TF, Bayik M (2006) Rituximab-CHOP versus CHOP alone in patients with diffuse large B cell lymphoma. Blood 108(11):259b–260b. https://doi.org/10.1089/pho.2006.24.637
Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, Lopez-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M, Grp M (2006) CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol 7(5):379–391. https://doi.org/10.1016/S1470-2045(06)70664-7
Horwitz SM, Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Byrd JC, Fayad LE, Fisher RI, Glenn MJ, Habermann TM, Harris NL, Hernandez-Ilizaliturri F, Hoppe RT, Kaminski MS, Kelsey CR, Kim YH, Krivacic S, LaCasce AS, Lunning M, Nademanee A, Press O, Rabinovitch R, Reddy N, Reid E, Roberts K, Saad AA, Sokol L, Swinnen LJ, Vose JM, Yahalom J, Zafar N, Dwyer M, Sundar H, Porcu P (2016) Non-Hodgkin's lymphomas, version 3.2016 featured updates to the NCCN guidelines. J Natl Compr Canc Ne 14(9):1067–1079. https://doi.org/10.6004/jnccn.2016.0117
Delarue R, Tilly H, Mounier N, Petrella T, Salles G, Thieblemont C, Bologna S, Ghesquieres H, Hacini M, Fruchart C, Ysebaert L, Ferme C, Casasnovas O, Van Hoof A, Thyss A, Delmer A, Fitoussi O, Molina TJ, Haioun C, Bosly A (2013) Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 14(6):525–533. https://doi.org/10.1016/S1470-2045(13)70122-0
Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ (2006) Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24(19):3121–3127. https://doi.org/10.1200/Jco.2005.05.1003
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2):185–229. https://doi.org/10.1124/pr.56.2.6
Hershman DL, McBride RB, Eisenberger A, Tsai WY, Grann VR, Jacobson JS (2008) Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin's lymphoma. J Clin Oncol 26(19):3159–3165. https://doi.org/10.1200/Jco.2007.14.1242
Gabizon A, Shmeeda H, Barenholz Y (2003) Pharmacokinetics of pegylated pegylated liposomal doxorubicin—review of animal and human studies. Clin Pharmacokinet 42(5):419–436. https://doi.org/10.2165/00003088-200342050-00002
Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A (2010) Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 10. doi: https://doi.org/10.1186/1471-2407-10-337
Wollina U, Dummer R, Brockmeyer NH, Busch JO, Kaatz M, Knopf B, Koch HJ, Hauschild A (2003) Multicenter study of pegylated pegylated liposomal doxorubicin in patients with cutaneous T-cell lymphoma. Cancer 98(5):993–1001. https://doi.org/10.1002/cncr.11593
Zaja F, Tomadini V, Zaccaria A, Lenoci M, Battista M, Molinari AL, Fabbri A, Battista R, Cabras MG, Gallamini A, Fanin R (2006) CHOP-rituximab with pegylated pegylated liposomal doxorubicin for the treatment of elderly patients with diffuse large B-cell lymphoma. Leuk Lymphoma 47(10):2174–2180. https://doi.org/10.1080/10428190600799946
Luminari S, Viel E, Ferreri AJM, Zaja F, Chimienti E, Musuraca G, Tucci A, Balzarotti M, Tani M, Salvi F, Pesce EA, Ferrari A, Liberati AM, Spadea A, Marino D, Bruno-Ventre M, Volpetti S, Bottelli C, Ravaioli E, Merli F, Spina M (2017) Nonpegylated pegylated liposomal doxorubicin combination regimen in patients with diffuse large B-cell lymphoma and cardiac comorbidity. Results of the HEART01 phase II trial conducted by the Fondazione Italiana Linfomi. Hematol Oncol. https://doi.org/10.1002/hon.2425
Combs S, Neil N, Aboulafia DM (2006) Pegylated liposomal doxorubicin, cyclophosphamide, and etoposide and antiretroviral therapy for patients with AIDS-related lymphoma: a pilot study. Oncologist 11(6):666–673. https://doi.org/10.1634/theoncologist.11-6-666
Iannitto E, Luminari S, Tripodo C, Mancuso S, Cesaretti M, Marcheselli L, Merli F, Stelitano C, Carella AM, Fragasso A, Montechiarello E, Ricciuti G, Pulsoni A, Paulli M, Franco V, Federico M (2015) Rituximab with cyclophosphamide, vincristine, non-pegylated pegylated liposomal doxorubicin and prednisone as first-line treatment for splenic marginal zone lymphoma: a Fondazione Italiana Linfomi phase II study. Leuk Lymphoma 56(12):3281–3287. https://doi.org/10.3109/10428194.2015.1029925
Katsuya H, Suzumiya J, Sasaki H, Ishitsuka K, Shibata T, Takamatsu Y, Tamura K (2009) Addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisolone therapy has a high risk of developing interstitial pneumonia in patients with non-Hodgkin lymphoma. Leuk Lymphoma 50(11):1818–1823. https://doi.org/10.3109/10428190903258780
Liote H, Liote F, Seroussi B, Mayaud C, Cadranel J (2010) Rituximab-induced lung disease: a systematic literature review. Eur Respir J 35(3):681–687. https://doi.org/10.1183/09031936.00080209
Wagner SA, Mehta AC, Laber DA (2007) Rituximab-induced interstitial lung disease. Am J Hematol 82(10):916–919. https://doi.org/10.1002/ajh.20910
Zayen A, Rais H, Rifi H, Ouarda M, Afrit M, Cherif A, Mezline A (2011) Rituximab-induced interstitial lung disease: case report and literature review. Pharmacology 87(5–6):318–320. https://doi.org/10.1159/000327681
Huober J, Schoch O, Templeton A, Spirig C, Thurlimann B (2010) Interstitial pneumonitis after treatment with bevacizumab and pegylated pegylated liposomal doxorubicin in a patient with metastatic breast cancer. Chemotherapy 56(1):69–70. https://doi.org/10.1159/000282286
Inaba K, Arimoto T, Hoya M, Kawana K, Nakagawa S, Kozuma S, Taketani Y (2012) Interstitial pneumonitis induced by pegylated liposomal doxorubicin in a patient with recurrent ovarian cancer. Med Oncol 29(2):1255–1257. https://doi.org/10.1007/s12032-011-9893-0
Mark M, Thurlimann B (2012) Fatal pneumonitis after treatment with pegylated pegylated liposomal doxorubicin in a patient with metastatic breast cancer in complete remission. Med Oncol 29(3):1477–1478. https://doi.org/10.1007/s12032-011-0002-1
Berenson JR, Yellin O, Kazamel T, Hilger JD, Chen CS, Cartmell A, Woliver T, Flam M, Bravin E, Nassir Y, Vescio R, Swift RA (2012) A phase 2 study of pegylated liposomal doxorubicin, bortezomib, dexamethasone and lenalidomide for patients with relapsed/refractory multiple myeloma. Leukemia 26(7):1675–1680. https://doi.org/10.1038/leu.2012.51
Blum KA, Jung SH, Johnson JL, Lin TS, Hsi ED, Lucas DM, Byrd JC, Cheson BD, Bartlett NL, CLG B (2010) Serious pulmonary toxicity in patients with Hodgkin's lymphoma with SGN-30, gemcitabine, vinorelbine, and pegylated liposomal doxorubicin is associated with an Fc gamma RIIIa-158 V/F polymorphism. Ann Oncol 21(11):2246–2254. https://doi.org/10.1093/annonc/mdq211
Del Conte G, Sessa C, von Moos R, Vigano L, Digena T, Locatelli A, Gallerani E, Fasolo A, Tessari A, Cathomas R, Gianni L (2014) Phase I study of olaparib in combination with pegylated liposomal doxorubicin in patients with advanced solid tumours. Brit J Cancer 111(4):651–659. https://doi.org/10.1038/bjc.2014.345
Mirza MR, Lund B, Lindegaard JC, Keldsen N, Mellemgaard A, Christensen RD, Bertelsen K (2010) A phase II study of combination chemotherapy in early relapsed epithelial ovarian cancer using gemcitabine and pegylated liposomal doxorubicin. Gynecol Oncol 119(1):26–31. https://doi.org/10.1016/j.ygyno.2010.06.022
Liu X, Hong XN, YJ G, Wang BY, Luo ZG, Cao JN (2008) Interstitial pneumonitis during rituximab-containing chemotherapy for non-Hodgkin lymphoma. Leuk Lymphoma 49(9):1778–1783. https://doi.org/10.1080/10428190802270886
Salmasi G, Li M, Sivabalasundaram V, Panzarella T, Tsang R, Kukreti V, Crump M, Kuruvilla J (2015) Incidence of pneumonitis in patients with non-Hodgkin lymphoma receiving chemoimmunotherapy with rituximab. Leuk Lymphoma 56(6):1659–1664. https://doi.org/10.3109/10428194.2014.963075
Huang YC, Liu CJ, Liu CY, Pai JT, Hong YC, Teng HW, Hsiao LT, Chao TC, Gau JP, Liu JH, Hsu HC, Chiou TJ, Chen PM, YB Y, Tzeng CH (2011) Low absolute lymphocyte count and addition of rituximab confer high risk for interstitial pneumonia in patients with diffuse large B-cell lymphoma. Ann Hematol 90(10):1145–1151. https://doi.org/10.1007/s00277-011-1268-2
Fridrik MA, Jaeger U, Petzer A, Willenbacher W, Keil F, Lang A, Andel J, Burgstaller S, Krieger O, Oberaigner W, Sihorsch K, Greil R (2016) Cardiotoxicity with rituximab, cyclophosphamide, non-pegylated pegylated liposomal doxorubicin, vincristine and prednisolone compared to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in frontline treatment of patients with diffuse large B-cell lymphoma: a randomised phase-III study from the Austrian Cancer Drug Therapy Working Group [Arbeitsgemeinschaft Medikamentose Tumortherapie AGMT](NHL-14). Eur J Cancer 58:112–121. https://doi.org/10.1016/j.ejca.2016.02.004
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, T., Shen, Q., Peng, H. et al. Incidence of interstitial pneumonitis in non-Hodgkin’s lymphoma patients receiving immunochemotherapy with pegylated liposomal doxorubicin and rituximab. Ann Hematol 97, 141–147 (2018). https://doi.org/10.1007/s00277-017-3160-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3160-1