Abstract
Cancer cachexia is defined as a state of involuntary weight loss, attributed to altered body composition with muscle mass loss and/or loss of adiposity. Identifying the association between cancer cachexia and outcomes may pave the way for novel agents that target the cancer cachexia process. Clinical parameters for measurement of cancer cachexia are needed. We conducted a single-institution retrospective analysis that included 86 NHL patients with the aim of identifying an association between cancer cachexia and outcomes in aggressive lymphomas using the cachexia index (CXI) suggested by Jafri et al. (Clin Med Insights Oncol 9:87–93, 15). Impact of cachexia factors on progression-free survival (PFS) and overall survival (OS) were assessed using log-rank test and Cox proportional hazards regression. Patients were dichotomized around the median CXI into “non-cachectic” (CXI ≥49.8, n = 41) and “cachectic” (CXI <49.8, n = 40) groups. Cachectic patients had significantly worse PFS (HR 2.18, p = 0.044) and OS (HR = 4.05, p = 0.004) than non-cachectic patients. Cachexia as defined by the CXI is prognostic in aggressive lymphomas and implies that novel therapeutic strategies directed at reversing cachexia may improve survival in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aggressive B-cell non-Hodgkin lymphomas (B-NHLs) typically present with constitutional symptoms including weight loss, fevers, and night sweats. While the presence of these symptoms has some prognostic utility [1], our growing experience with metabolic disorders as they relate to the pathogenesis and natural history of lymphoma has led us to believe that the “cancer cachexia syndrome,” a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass [2], may be the more relevant prognosticator in this regard. Cachexia represents a state of involuntary weight loss and altered body composition attributed to the effects of host inflammatory response, inflammatory cytokines, and tumor bi-products [3, 4]. One of the prevailing hypotheses on this phenomenon concerns the tumor’s pronounced demand for metabolic building blocks and ability to reprogram energy metabolism (e.g., the Warburg effect) to meet this need [5, 6]. These changes include alterations in metabolism of fat, protein, and carbohydrates with resultant muscle wasting or sarcopenia and fat loss or adipopenia [7]. Mechanistically, the basis for this systemic recruitment for these metabolic building blocks remains inadequately understood. Most would agree on some contribution of inflammation to the process of cachexia, with additional hallmarks of this disorder including elevated levels of C-reactive protein (CRP) [8], anemia, and hypoalbuminemia [9]. However, the interplay between inflammation, other potential biologic mechanisms, the development of cachexia and the pathogenesis of cancers, including lymphomas, has yet to be defined.
Several prognostic factors have been validated for aggressive NHL patients, including, the International Prognostic Index (IPI) which takes into account the age, ECOG performance status, stage, LDH levels, extranodal involvement, and stage of disease in the patient [10]. Diffuse large B-cell lymphoma (DLBCL) has had additional parameters specifically defined, including the presence of c-myc translocation while harboring a concurrent bcl-2 and/or bcl-6 mutation [11] and cell of origin [12]. Although each of the aforementioned measurements are independently prognostic of outcome, they have yet to achieve predictive utility.
Based on our current knowledge of mechanisms underlying cancer cachexia, several of these may represent therapeutically targetable pathways that are both reflective of pathogenesis of disease and predictive of outcomes in lymphoma. In fact, investigators have shown a correlation between various protein factors pertinent to cachexia and clinical outcomes in lymphoma. For example, elevated levels of leptin, which typically suppress hunger, have been correlated with poor survival in aggressive lymphoma subsets, attributed to increased activation of proliferative signals via the PI3K/AKT pathway [13]. Similarly, NFκB activation, a protein intermediate dominant in inflammation, is typically associated with aggressive phenotypes of lymphoma usually characterized by the presence of constitutional symptoms and chemo-resistance [14]. These suggested links between cachexia and lymphoma biology imply a potential role for cachexia surveillance for prognostication and pharmacotherapeutic manipulation of the cachexia axis to improve the management of lymphoma.
More recently, an objective formula that measures cachexia, specifically a cachexia index (CXI), was proposed by Jafri et al., incorporating three measured variables—a radiographically derived skeletal muscle index (SMI), albumin (alb), and the neutrophil to lymphocyte ratio (NLR)—was identified as a prognostic tool in cancer [15]. Our aim for the current study was to evaluate cachexia measures, including the aforementioned CXI for their potential prognostic utility in aggressive NHL including DLBCL and mantle cell lymphoma (MCL).
Materials and methods
This was a retrospective study that reviewed all patients diagnosed with DLBCL and MCL between 1991 and 2015 and managed at our institution. Diagnostic surgical pathology was reviewed to confirm NHL subtype. We identified 239 cases fitting this criteria. Patients who did not have baseline imaging of high quality available in our electronic imaging database for measures of muscle indices (as described below), were excluded. Ultimately, 86 patients were identified with available imaging and were analyzed to assess for associations between cachexia and clinical outcomes.
Patient characteristics including age, height, weight, and body mass index (BMI) at the time of diagnosis were collected. Laboratory tests done within a window of 1 week prior to date of diagnosis and up to the date of treatment were reviewed for the following: LDH, lymphocyte percentage, absolute lymphocyte count, absolute neutrophil count, albumin, and complete blood counts. Diagnostic surgical pathology and cytogenetic reports including fluorescent in situ hybridization (FISH) and molecular pathology were reviewed to obtain information status cell of origin by Hans criteria [16], c-myc, bcl-2, and bcl-6 status.
Patient pre-treatment computed tomography (CT) scan and positron emission tomography (PET) scans were reviewed. CT muscle indices for evaluating sarcopenia were recorded [17]. For each CT scan, axial images from the third lumbar vertebrae (L3) was assessed. Images were analyzed with Slice-O-Matic V4.3 software (Tomovision), which enabled specific tissue demarcation using Hounsfield unit (HU) thresholds as defined by Camus et al. 2014 [18]. The tissue boundaries for muscle in the L3 region were manually annotated and cross-sectional areas (cm2) were computed and normalized for stature and reported as a skeletal muscle index (SMI, cm2/m2). Sarcopenic patients were defined as those whose SMI fell below the median muscle mass measured for men/women in our population per prior reports [18, 19].
Patients were assessed for presence or absence of cachexia utilizing this CXI index, defined as SMI/Alb × NLR [15]. Although there is a clear lack of consensus of how to define cachexia, the most common practice has been to define cut-offs around median values for sarcopenia and adipopenia as measures of cachexia, in various cohorts of patients with solid and hematologic malignancies [17–19]. We applied this approach to the CXI: “cachectic” and “non-cachectic” patients in our cohort were defined as having a CXI <49.8 versus CXI ≥49.8, respectively around the median value of CXI for our population.
Overall survival (OS) was defined as the time between the date of diagnosis and death or date of last known follow-up. Progression-free survival (PFS) was defined as time between the date of diagnosis and the date of disease relapse or progression. The impact of the CXI on PFS and OS were estimated using log-rank test and Cox proportional hazards regression.
Results
Eighty-six patients with quality radiographic images were analyzed. Patient characteristics are summarized in Table 1. Treatments administered to patients are also shown in Table 1 with the majority of DLBCL patients (n = 67, 88%) having received R-CHOP (Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. Treatment strategies were more variable in the MCL cohort (n=10, 12%); patients were treated either with R-CHOP ± botezomib (three patients, 30%), rituximab ± bortezomib (two patients, 20%), hyperCVAD ± bortezomib (cyclophosphamide, doxorubicin, vincristine, and prednisone alternating with high-dose methotrexate and cytarabine, three patients, 30%), BR (Bendamustine/Rituximab, one patient, 10%) or observed (one patient, 10%). Divided around the median SMI, 43 (50%) of our patients had muscle loss. The cachexia index could be calculated in 81 patients; divided around the median CXI, 41 (50.6%) patients had a CXI ≥49.8 (non-cachectic) and 40 (49.3%) patients had a CXI <49.8 (cachectic).
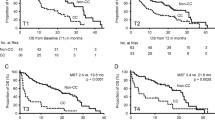
With a median follow-up time of 59.5 months, OS was 76% and PFS was 66% for the cohort. On univariate analysis, patients with a low SMI (below the median) had similar survival outcomes as compared to those with a high SMI (PFS, HR 0.64, p = 0.23; OS, HR 0.87, p = 0.74). There was no difference in clinical outcomes for those patients who were underweight versus those with normal or increased BMI. However, using the CXI, patients with “cachexia” had significantly worse PFS (HR 2.18, p = 0.044) and OS (HR = 4.05, p = 0.004) than those with “no cachexia” (Fig. 1). Low albumin (<3.5 g/dL), advanced stage, and elevated LDH also had significant impacts on OS (HR = 3.19, p = 0.011; HR = 5.77, p = 0.008; HR = 3.87, p = 0.009, respectively). On multivariate analysis (MVA) adjusting for LDH, stage, and CXI, the presence of cachexia by CXI did not impact PFS (HR = 1.67, p = 0.2) but was associated with a worse OS (HR = 3.11, p = 0.032; Table 2). Elevated LDH also adversely impacted OS (Table 2).
Discussion
Cancer cachexia is a multifactorial syndrome primarily defined by the 2007 cachexia consensus conference as “a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass” [2]. Historically, cachexia was thought of as a syndrome of anorexia, fatigue, and weight loss. However, it was recently redefined precisely as the lean muscle mass loss associated with chronic illness and/or cancer [17]. There is a growing body of literature to suggest that better understanding of this process as it relates to lymphoma may enable us to identify prognostic and predictive tools in aggressive NHL.
Investigators recently published results of a retrospective analysis of the prognostic impact of cachexia on DLBCL survival in 80 patients [18]. Forty-four (55%) and 46 (58%) of patients were considered sarcopenic or adipopenic, respectively. In this study, a difference was demonstrated in the median PFS of 13.6 months in the adipopenic group versus 49.4 months in the non-adipopenic group (hazard ratio (HR) = 2.27; 95% confidence interval (CI) 1.3–4; p = 0.0042). What was striking was that 2-year OS in the sarcopenic population was 46% compared with 84% in the non-sarcopenic group (HR = 3.22; 95% CI = 1.73–5.98; p = 0.0002). This is inconsistent with our findings showing a lack of correlation between sarcopenia based on SMI and survival. This discrepancy could be explained by the difference in median age in the Camus study [18] versus our own (78.8 years versus 64 years, respectively), anticipating a higher prognostic capacity for SMI in older patients. Further investigation of SMI with a larger sample size might also result in more significant findings.
Jafri and colleagues [15] proposed a formula for an index that measures cancer cachexia, i.e., the CXI, in a more detailed manner rather than solely referring to simple measures such as weight or BMI. The CXI utilizes three parameters individually linked to poor outcomes, namely the SMI [18], albumin levels [20], and neutrophil-to-lymphocyte ratio [21], and was shown to be predictive of poor outcomes in lung cancer patients.
In a similar manner, we applied the CXI to aggressive lymphoma and found correlations between a low CXI and survival. Univariate analysis showed a significant decrease in PFS (HR= 2.18, p = 0.044) and OS (HR=4.05, p = 0.004) in patients with cachexia, defined as a CXI below the median threshold in our population. The presence of a low CXI increased the risk of death threefold on MVA as well (HR = 3.11, p = 0.032). As a retrospective single-institution study, there are inherent biases that limit clear interpretation of these results including patient selection based on availability of baseline imaging. More importantly, another limitation of our study includes the lack of well-defined cut-offs for CXI, common to other measures of cachexia such as sarcopenia and/or adipopenia. It is expected that the CXI may be influenced by sex, race, diet, lifestyle, age, and perhaps geographic distribution. This supports the need for a prospective multi-institutional effort to validate the CXI (and define cut-offs) as a prognostic tool applicable to diverse populations.
A number of correlations between biological mechanisms pertinent to cachexia and clinical outcomes in lymphoma have been described in the literature and support our findings. For example, elevated levels of leptin, a hunger suppressant, are associated with poor survival in aggressive lymphoma subsets, attributed to increased activation of proliferative signals via the PI3K/AKT pathway [13]. Alternatively, polymorphisms in ghrelin, a circulating orexin and antagonist of leptin, has been associated with a decreased incidence of aggressive subsets of lymphomas [22]. Links have also been identified between proteins of inflammation, (tumor necrosis factor- α (TNF-α), interleukin-6 (IL-6), IL-10), members of the insulin and insulin-like growth factor axes and myokines, (vascular endothelial growth factor (VEGF)), and aggressive phenotypes of lymphoma usually characterized by the presence constitutional symptoms and chemo-resistance [14, 23–28]. Our results suggest that the CXI may be a way to screen for the disruption of such biologic mechanisms in a way that could be applied as predictive tools to guide our therapeutic strategies.
Strategies to target cancer cachexia are in early stages of development and include ghrelin receptor agonists (such as anamorelin), metformin, selective androgen receptor modulators (such as GTx-024), and anti-myostatin antibodies. For instance, a study by Oliveira et al. found that metformin minimized a tumor-induced wasting state by reducing the activity of proteolytic enzymes responsible for the anabolism of muscle protein [29]. Currently, there are no clinical parameters which identify lymphoma patients who would be most likely to benefit from such new treatment strategies. The relationship we demonstrate between cachexia and survival using the CXI as a clinical parameter of cachexia would suggest that utilizing such novel agents in patients demonstrating a low CXI may help to improve clinical outcomes and warrants investigation in clinical trial.
References
Shipp MA (1994) Prognostic factors in aggressive non-Hodgkin’s lymphoma: who has “high-risk” disease? Blood 83(5):1165–1173
Evans WJ, Morley JE, Argilés J et al (2008) Cachexia: a new definition. Clin Nutr 27(6):793–799
Staal-van den Brekel AJ, Dentener MA, Schols AM, Buurman WA, Wouters EF (1995) Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol 13(10):2600–2605
Kuroda K, Nakashima J, Kanao K et al (2007) Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology 69(1):113–117
Demetrius LA, Coy JF, Tuszynski JA. (2010) Cancer proliferation and therapy: the Warburg effect and quantum metabolism. Theor Biol Med Model. 7:2. doi:10.1186/1742-4682-7-2
Fredrix EW, Soeters PB, Wouters EF, Deerenberg IM, von Meyenfeldt MF, Saris WH (1990) Energy balance in relation to cancer cachexia. Clin Nutr 9(6):319–324
Petruzzelli M, Wagner EF (2016) Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev 30(5):489–501
Falconer JS, Fearon KC, Plester CE, Ross JA, Carter DC (1994) Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg 219(4):325–331
Ohnuma T. 2003 Manifestations of Cachexia. Holland-Frei Cancer Medicine. Hamilton
Project TIN-HsLPF (1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The international non-Hodgkin’s lymphoma prognostic factors project. N Engl J Med 329:987–994
Perry AM, Alvarado-Bernal Y, Laurini JA et al (2014) MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab. Br J Haematol 165(3):382–391
Lenz G, Wright G, Dave SS et al (2008) Lymphoma/leukemia molecular profiling project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 359(22):2313–2323
Uddin S, Bu R, Ahmed M, Hussain AR, Ajarim D, Al-Dayel F et al (2010) Leptin receptor expression and its association with PI3K/AKT signaling pathway in diffuse large B-cell lymphoma. Leukemia & Lymphoma 51(7):1305–1314
Carbone A, Gloghini A, Kwong Y-L, Younes A (2014) Diffuse large B cell lymphoma: using pathologic and molecular biomarkers to define subgroups for novel therapy. Ann Hematol 93(8):1263–1277
Jafri SH, Previgliano C, Khandelwal K, Shi R (2015) Cachexia index in advanced non-small-cell lung cancer patients. Clin Med Insights Oncol 9:87–93
Hans CP, Weisenburger DD, Greiner TC et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282
Prado CM, Sawyer MB, Ghosh S et al (2013) Central tenet of cancer cachexia therapy: do patient with advanced cancer have exploitable anabolic potential? Am J Clin Nutr 98:1012–1019
Camus V, Lanic H, Kraut J et al (2014) Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur J Haematol 93(1):9–18
Lanic H, Kraut-Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM, Stamatoullas A, Leprêtre S, Tilly H, Jardin F (2014) Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma 55(4):817–823
Gupta D, Lis CG (2010) Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 9:69
Lin GN, Peng JW, Liu PP, Liu DY, Xiao JJ, Chen XQ (2014) Elevated neutrophil-to-lymphocyte ratio predicts poor outcome in patients with advanced non-small-cell lung cancer receiving first-line gefitinib or erlotinib treatment. Asia Pac J Clin Oncol. doi:10.1111/ajco.12273. [Epub ahead of print]
Skibola CF, Curry JD, Nieters A (2007 Jul) Genetic susceptibility to lymphoma. Haematologica 92(7):960–969
Charbonneau B, Maurer MJ, Ansell SM et al (2012) Pretreatment circulating serum cytokines associated with follicular and diffuse large B-cell lymphoma: a clinic-based case-control study. Cytokine 60(3):882–889
Bonetto A, Aydogdu T, Jin X et al (2012) JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab 303(3):E410–E421
Bonetto A, Penna F, Aversa Z et al (2013) Early changes of muscle insulin-like growth factor-1 and myostatin gene expression in gastric cancer patients. Muscle Nerve 48(3):387–392
Karmali R, Dalovisio A, Borgia JA et al (2015) All in the family: clueing into the link between metabolic syndrome and hematologic malignancies. Blood Rev 29(2):71–80
Karmali R, Paganessi LA, Frank RR, Jagan S, Larson ML, Venugopal P, Gregory SA, Christopherson KW 2nd (2013) Aggressive disease defined by cytogenetics is associated with cytokine dysregulation in CLL/SLL patients. J Leukoc Biol (1):161–70
Vishwamitra D, Shi P, Wilson D et al (2011) Expression and effects of inhibition of type 1 insulin-like growth factor receptor tyrosine kinase in mantle cell lymphoma. Heamatolgica 96(6):871–880
Oliveira AG, Gomes-Marcondes MC (2016) Metformin treatment modulates the tumour-induced wasting effects in muscle protein metabolism minimising the cachexia in tumour-bearing rats. BMC Cancer 16:418. doi:10.1186/s12885-016-2424-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Karmali, R., Alrifai, T., Fughhi, I.A.M. et al. Impact of cachexia on outcomes in aggressive lymphomas. Ann Hematol 96, 951–956 (2017). https://doi.org/10.1007/s00277-017-2958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2958-1