Abstract
Central venous catheters are a leading cause of upper-extremity deep vein thrombosis. Concomitant severe thrombocytopenia makes anticoagulation for catheter-related thrombosis (CRT) in patients with acute leukemia (AL) a challenge. Incidence of CRT has been reported to be increased in those with peripherally inserted central catheters (PICC) vs. those with centrally inserted ones (CICC). Our objective is to compare the incidence rate of CRT in leukemia inpatients who received either a PICC vs. CICC. We retrospectively reviewed adult inpatients admitted to hematology wards with a new diagnosis of AL and who received either a PICC or a CICC. Baseline patient and catheter characteristics were recorded. Our primary outcome was the incidence rate of CRT in each group. The secondary outcomes included rates of infectious and mechanical complications. Six hundred sixty-three patients received at least one PICC (338) or CICC (325) insertion. A total of 1331 insertions were recorded, with 82 (11.7 %) and 41 (6.5 %) CRT in the PICC and CICC groups, respectively. The incidence rates were 1.89 and 0.52 per 1000 catheter day in the PICC and CICC groups, respectively. A PICC, when compared to CICC, was a significant risk factor for CRT (sHR 2.5, p < 0.0001). The prevalence and incidence rates of CRT in our AL patients were higher than predicted for a general cancer patient population. These rates were higher in the PICC group compared to the CICC group. We recommend careful consideration of thrombotic and bleeding risks of AL inpatients when choosing a central venous catheter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE) is a common disease associated with potentially fatal complications and both acute and chronic morbidities [1, 2]. Its incidence is increased in patients with active malignancy, receiving chemotherapy, and who require hospitalization for acute medical illness [3, 4]. Central venous catheters for administration of chemotherapy, antibiotics, and blood products also increase the risk of upper-extremity deep vein thrombosis (DVT), often referred to as catheter-related thrombosis (CRT) [5]. The reported incidence of CRT varies depending on the type of cancer and type of central venous catheter (CVC) used. Central catheters are either peripherally inserted (PICC) or centrally inserted (CICC). Most studies have reported significantly lower incidence rates of CRT in cancer patients who received CICC when compared to PICC across different types of cancer [6–10]. In hematological malignancies, the incidence rates of CRT are reported to be 5.8–40 or 1.7–10.2 % in patients who received either a PICC or CICC, respectively [11–14]. One study retrospectively reported a prevalence rate of 5.9 % in acute leukemia patients who received CICC [15]. Limitations of the available data include small numbers of patients studied, few studies specifically looking at acute leukemia and no comparative studies between PICC and CICC.
The standard treatment for VTE is anticoagulation. This is complicated for patients with acute leukemia as both the disease as well as the treatment can induce severe thrombocytopenia. Due to the need for chemotherapy, blood transfusion, and antibiotic, all leukemia patients require prolonged central venous access. This creates a unique population of patients at higher risk for both thrombotic and hemorrhagic events. Patients with severe thrombocytopenia have been excluded from VTE treatment studies. Management is thus highly dependent on the physician clinical judgment or expert opinion [16]. Reducing the risk of VTE will help decrease the need to make difficult anticoagulant decisions in thrombocytopenic patients.
This is a retrospective, multicenter study aimed to compare the prevalence and incidence rates of CRT in acute leukemia patients who either received a PICC or a CICC during their chemotherapy treatment. We have also examined the rates of recurrent CRT, noncatheter-related VTE, as well as catheter-related infectious and mechanical complications as secondary outcomes.
Methods
Study design
This was a retrospective study of patients admitted to hematology wards with acute leukemia. The inclusion criteria were (1) adult patients (>17 years old); (2) admitted with a new diagnosis of acute leukemia between January 1, 2002, and December 31, 2013; and (3) must have received a CVC, either a PICC or CICC. Patients were identified from the medical records of involved hospitals based on ICD-10CA codes for acute leukemia. Patients were further classified based on Canadian Classification of Health Intervention codes for insertion of a PICC or CICC. Patients were divided into one of two groups, as either PICC or CICC, based on their first insertion. All subsequent CVC insertions were recorded for each patient, and each insertion represented an independent data point for outcome analysis. As described below, we have accounted for confounding factors (such as repeated insertions in the same patients) in our analyses, with the primary outcome being specific to first insertions. Follow-up duration for each patient represented the catheter dwell time. Three medical centers in the province of Alberta, Canada, were included in the study. The University of Alberta Hospital (Edmonton) utilized mainly PICC, whereas Foothills and Peter Lougheed Medical Centers (Calgary) employed CICC for leukemia inpatients. Ethics approvals from both cities were obtained (Edmonton Pro00051738; Calgary REB14-1916), in keeping with the Canadian Tri-Council Policy statement on ethical conduct for research.
Primary outcome
The primary outcome was to compare the prevalence and incidence rates of CRT associated with PICC vs. CICC insertions in leukemia patients. Each CVC insertion was treated as an independent data point, starting with the first catheter received during the induction chemotherapy and followed in time for subsequent admissions including consolidation chemotherapy and hematopoietic stem cell transplant (HSCT). All of the CRTs recorded were symptomatic, and further investigations were ordered by the medical team after high clinical suspicion. The involved centers did not have policies for routine imaging to screen for asymptomatic CRT, and incidental CRTs were not included in the study. CRT was defined as formation of thrombus in the vein or connected vein of the inserted catheter as confirmed by imaging (Doppler ultrasonography or other appropriate modalities such as venogram). Any thrombus detected from the time of insertion and up to 5 days of catheter removal was included. An occluded catheter alone was not sufficient to make the diagnosis.
Secondary outcomes
These include recurrent CRT as well as noncatheter-related VTE such as pulmonary embolism (PE), lower extremity DVT, or thrombosis in other venous systems. Lower-extremity VTE was defined as the presence of a thrombus in the popliteal vein or more proximal veins as seen on compression Doppler ultrasonography. Diagnosis of PE was confirmed by the presence of a thrombus in a segmental or more proximal pulmonary artery as seen on computed tomography pulmonary angiography or ventilation-perfusion scan. Recurrent CRT was defined as recurrence of thrombus in the same venous system as the catheter (without removal) after clearly documented complete resolution on imaging or objectively documented extension of the original thrombus compared to previous imaging.
Catheter-related bacteremia and mechanical complications were recorded as secondary outcomes. Catheter-related infection was defined as a positive blood culture with evidence of colonized catheter tip (with respective cultures growing same organisms). All-cause bacteremia was defined as any blood cultures growing bacterial pathogens with or without catheter tip colonization. Cultures growing coagulase-negative staphylococci in only one of multiple sets of cultures were excluded to avoid uncertainty of clinical significance. Mechanical complications were categorized as occlusion, dislodgment, or leakage.
Statistical analysis
Continuous variables were summarized as median with interquartile range (IQR) and categorical variables were described by frequency distributions. The chi-squared test or Fisher’s exact test (if cell counts <5) were employed to assess the association between two categorical variables. The Wilcoxon-Mann-Whitney test (for two groups) was used to compare continuous variables. Incidence rates for primary and secondary outcomes were calculated using person-time approach. Competing risks regression model as described by Fine and Gray 1999 [17] was used to calculate the subhazard ratios (sHRs) in order to determine the effect of variables on each of the primary and secondary outcomes, while accounting for death as a competing risk factor. Death was shown to be a significant competing risk factor in AML patients in a different study [18]. This method accounted for catheter indwelling time as well. Univariable and multivariable analyses were performed, and sHRs (with 95 % Confidence Interval) were presented for each outcome. Variables that were included in the model include type of leukemia, age, gender, other active cancer, body mass index (BMI) greater than 30, smoking history, surgery, diabetes, heart failure, hypertension, inflammatory bowel disease (IBD), use of hormonal replacement, genetic thrombophilia, pregnancy, previous history of VTE, city of insertion, and number of repeated insertions per patient. Cumulative incidence of each outcome, accounting for death as a competing risk factor, was plotted by catheter type. Two-sided p values of <0.05 were considered as statistically significant. All analyses were performed using STATA statistical software (version 13, StataCorp, TX, USA [19]).
Results
Baseline characteristics
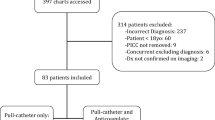
Charts for 808 consecutive, newly diagnosed AL patients were reviewed, with 145 patients excluded as they did not receive a CVC. Data for 663 were included, of whom 338 had a PICC and 325 had a CICC as a first insertion. Baseline characteristics are summarized in Table 1. The majority of patients (>90.0 %) were diagnosed with either acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL). Other types of leukemia included acute promyelocytic leukemia (APL) and acute leukemia of unspecified cell type. Fifty-four percent of the patients were male, and certain baseline cardiovascular risk factors were different between the two groups (Table 1).
Catheter-related characteristics are summarized in Supplemental: Table S1. The median dwell time of catheters were 38 days (IQR 15.5–90.0) and 98 days (IQR 37.0–174.5) in the PICC and CICC groups, respectively (p < 0.0001). The most common indication for insertion for both catheter types was for chemotherapy. The most common indication for removal for both catheter groups was for completion of treatment, followed by clinically suspected infection. The PICCs were replaced more frequently (47.0 % of all PICC insertions) than the CICCs (29.0 % of all CICC insertions) (p < 0.0001), with infection being the most common reason for replacement (Supplemental: Table S1). Furthermore, given the long inclusion period (2002–2013), two different manufacturers of PICC were implemented among our patient population. To account for this, correlation of the prevalence of CRT between the two periods was determined to be statistically insignificant (p = 0.16).
Primary outcome
Catheter-related thrombosis
The number of patients who developed CRT in PICC and CICC were 77/338 (22.8 %) and 40/325 (12.3 %), respectively (p = 0.001). When the first CVC insertions were considered, the number of patients who developed CRT in PICC and CICC were 48 (14.8 %) and 21 (6.5 %). The number of CRT that developed within a month of catheter insertion is 49/77 (63.6 %) and 19/40 (47.5 %) in the PICC and CICC groups, respectively (p = 0.03). Supplemental: Table S2 summarizes the rates of CRT according to the order of insertion or time course of CRT. Overall, 51 (66.2 %) and 22 (55.0 %) of the patients who developed a CRT were thrombocytopenic with a platelet count <50 × 109/L at the time of their diagnosis in the PICC and CICC groups, respectively (p = 0.7). The number of patients who developed CRT and received a therapeutic dose of anticoagulation in the PICC and CICC groups was 35 (45.5 %) and 25 (62.5 %), respectively (p = 0.06). Only two and one cases of CRT occurred in patients already treated with anticoagulation after a previous CRT in the two respective groups (p = 0.2). The incidence rates of CRT in PICC and CICC groups were 1.9 per 1000 catheter day (95 % CI 1.5–2.3) and 0.5 per 1000 catheter day (95 % CI 0.4–0.7, p < 0.0001), respectively.
Secondary outcomes
A summary of prevalence and incidence rates of all outcomes are presented in Tables 2 and 3 (as per the total number of CVC insertions; N = 1331).
Recurrent catheter-related thrombosis
Out of the seven and four patients who developed recurrent CRT, five and two occurred within 30 days of the previous incidence, in the PICC and CICC groups, respectively (p = 0.3).
Concurrent venous thromboembolic events
Overall, there were 17, 16, and 2 cases of lower-extremity DVT, PE, and portal venous thrombosis, respectively. The rates of DVT and PE were similar between the PICC (8 and 9) and the CICC (9 and 7) groups, respectively (p = 1.0 and 0.5). Fourteen and 13 patients developed a VTE within 90 days of the catheter insertion date in the PICC and CICC groups, respectively (p = 0.2).
Catheter-related and all-cause bacteremia
Although the prevalence of bacteremia was higher in the CICC group, the incidence rate per 1000 catheter days was not significantly different (Tables 2 and 3). The five most common organisms isolated were Staphylococcus group (Staphylococcus aureus/coagulase-negative), Escherichia coli, Klebsiella pneumonia, Enterococcus faecium, and Streptococcus mitis. Patients who developed bacteremia during HSCT accounted for 6/74 (8.0 %) and 49/137 (36.0 %) of the PICC and CICC groups, respectively (p < 0.0001). Only two and five deaths were related to sepsis in the PICC and CICC groups, respectively (p = 0.3).
Catheter-related mechanical complications
The most common mechanical complication (>75.0 %) in both groups was catheter malfunction/dislodgement (p = 0.06), followed by catheter occlusion and leakage.
Univariable and multivariable competing risks regression model
Catheter-related thrombosis
A univariable competing risks analysis was performed to evaluate for predictors of CRT accounting for death as a competing risk factor. Catheter type (PICC with sHR 2.5, p < 0.0001), a diagnosis of APL (sHR 1.9, p = 0.04), as well as repeated catheterizations per patient (2–5 and >5 insertions per patients with sHR 2.9 and 4.0, respectively, p < 0.0001) were found to be significant risk factors of CRT. A cutoff for p value <0.05 was used to avoid overlooking certain factors in the multivariable analysis. In this model, only catheter type (sHR 2.2, p < 0.0001) and repeated catheterizations per patient (2–5 and >5 insertions per patients with sHR 2.8 and 3.3 with p < 0.0001 and p = 0.001, respectively) remained significant predictors of CRT in this patient population. Figure 1 summarizes the sHR for all of the factors in the univariable analysis of CRT.
Univariable competing risks regression model of factors affecting catheter-related thrombosis, accounting for death as a competing risk factor. Certain variables (e.g., surgery, genetic thrombophilia, pregnancy, and HSCT) did not have enough data to be analyzed in the model. AML acute myeloid leukemia, ALL acute lymphoblastic leukemia, APL acute promyelocytic leukemia, BMI body mass index, CHF congestive heart failure, IBD inflammatory bowel disease, OCP/HRT oral contraceptive pill/hormonal replacement therapy, VTE venous thromboembolism
This analysis was repeated excluding those patients who developed a CRT in their first insertion, in order to account for the increased risk of CRT in those with a previous history. Catheter type (PICC sHR 2.4, p = 0.002) and repeated catheterizations per patient (3–5 and >5 insertions per patient with sHR 5.0 and 6.3 respectively, p < 0.0001) remained significant in the final model of the multivariable analysis.
Secondary outcomes
Similar analyses were also performed for all secondary outcomes. The multivariable analysis indicated that IBD (sHR 11.5, p = 0.02) and the use of oral contraceptive pills or hormonal replacement therapy (OCP/HRT, sHR 4.3, p = 0.04) remained significant predictors for recurrent CRT (Supplemental: Table S3). Similarly, a BMI greater than 30 (sHR 2.9, p = 0.005) and a history of previous VTE (sHR 2.9, p = 0.05) remained significant risk factors for the concurrent development of other VTE (PE/DVT). With regard to all-cause bacteremia, HSCT (sHR 8.8, p < 0.0001), diabetes (sHR 1.5, p = 0.04), and repeated catheterizations per patient (2–5 and >5 repeated insertions with sHR 2.6 and 3.5, respectively, p < 0.0001) remained significant predictors of this outcome in multivariable analysis. Catheter type (PICC with sHR 2.3, p < 0.0001) was the only predictor of catheter-related mechanical complications.
Discussion
In this study, the prevalence rate of CRT in acute leukemia patients who received a PICC (22.8 %) was twofold higher than those with a CICC (12.3 %). Although the difference remains the same between the two groups, the actual rate of CRT per group is lower if first insertions only were considered (14.8 vs. 6.5 %, respectively; p = 0.001). These rates are higher than those reported in studies of various cancer patient population (PICC; CICC 4.5–6.9 %; 1.2–8.7 % [6–9, 20, 21]). There was no difference in rates of recurrent CRT and other VTEs between the two groups. In patients with hematological cancers, the prevalence rates of CRT in PICC and CICC are reported as 5.8–40 and 1.7–10.2 %, respectively [9, 11, 12, 14, 22, 23]. The variation in the rates is often attributed to differences in the use of thromboprophylaxis, in the small number of patients enrolled, and in defining and diagnosing CRT. In fact, those rates are significantly increased if screening for CRT is performed more frequently, 12.0–51.0 % [10, 24, 25]. One study with a similar patient population (acute leukemia) reported a 5.9 % rates of CRT in patients who received CICC [15]. The reasons for higher rates of CRT in hematological-oncology patients who received a CVC may be due to the prolonged use of chemotherapy and the nature of the cancer itself. The importance of our study lies in the harm reduction benefits of using CICC over PICC in order to reduce CRT in AL patients. This is particularly important given the uncertainty involving anticoagulation use to treat CRT in AL patients who are commonly thrombocytopenic [16].
The majority of the catheter-related thromboses occurred acutely in the ipsilateral side of insertions. This is in keeping with the proposed mechanism of CRT [5]. Other catheter characteristics that may increase risk of CRT were difficult to ascertain given that the majority of these CVCs were of certain size, number of lumens, and cannulated vein. Disease and patient characteristics were not shown to significantly affect CRT, however, PICC catheter type and repeated catheterizations per patient remained significant in the multivariable analysis. In our repeat competing risks regression analysis, we excluded CRT that developed in first CVC insertions, in order to eliminate the presumed increased risk of CRT in those with a previous history. This was demonstrated in a different study of patients requiring hemodialysis catheter that showed increased rates of CRT from 14 to 47 % from the first to the third insertion, respectively [26]. In our study, 3–5 and greater than 5 insertions per patient were associated with increased risk of CRT (sHR 5.0 and 6.3, respectively). In another study, diabetes (HR = 3.2), metastatic cancer (HR = 3.3), and COPD (HR = 2.7) increased the risk of CRT in patients with PICC [27].
In this study, IBD and the concomitant use of OCP/HRT increased the risk of recurrent CRT, in keeping with previously published studies in the general population [28–30]. Furthermore, previous history of VTE and BMI greater than 30 increased the risk of other VTE (including PE and DVT). Previous VTE is a well-established risk factor for recurrent episodes [31, 32]. Increased BMI is roughly estimated to increase risk of VTE by twofold [33, 34]. These factors may aid in VTE risk stratification of AL patients leading to more close monitoring for signs and symptoms of VTE or initiation of thromboprophylaxis.
In addition to thrombotic complications, this study examined infectious catheter-related complications. The prevalence rates for catheter-related and all-cause bacteremia were consistently higher in CICC than the PICC. The rates we report are closer to a study in patients receiving HSCT and are consistent with several reports in the literature [35, 36]. In general, the rates of CVC bacteremia ranged from 0.0-20.8 % [37]. Moreover, the vast majority of all-cause bacteremia in our study occurred in the CICC group, where HSCT was a significant risk factor in the multivariable analysis (sHR = 8.8, p <0.0001). This is likely due to the prolonged and intensive chemotherapy regimen given prior to the HSCT, causing weakening of the gastrointestinal wall mucosa. Another factor that increased the risk of all-cause bacteremia in this study was a diagnosis of diabetes (sHR = 1.5, p = 0.04). It is well established that hyperglycemic states affect the function of the neutrophil as well as diminish peripheral blood circulation [38].
There are some important limitations to consider. This is a retrospective study which may be confounded by incomplete or missing information, as well as subjective bias in diagnosis. Institutional preference seems to be the predominant reason for the difference in using PICC vs. CICC between the two cities. Furthermore, there is a significant difference in the catheter dwell time between the two catheter types. However, given that most of the CRT occurred within 90 days of catheter insertion, durations longer than this time can be considered insignificant to the development of CRT. Additionally, the competing risk regression analysis employed the insertion and removal dates for each catheter in order to account for this limitation. To minimize selection bias, we have included all consecutive patients at their respective center. There are some significant differences between the two patient groups, in particular with respect to their gender, cardiovascular risk, and smoking history. However, we believe that we have accounted for these differences in the multivariable analysis.
Conclusions
This retrospective study demonstrated a higher risk of CRT in AL patients who received a PICC as compared to a CICC. This is potentially of major importance, given the potential bleeding risks associated with anticoagulation in this severely thrombocytopenic population and the inherent risks associated with not anticoagulating these patients. The prevalence and incidence rates of CRT in our AL patients were higher than predicted for a general cancer patient population. These rates were higher in the PICC group compared to the CICC group. We, therefore, recommend weighing the thrombotic risks against those of bleeding in AL patients undergoing intensive chemotherapy to aid in choosing a less thrombogenic CVC such as a CICC rather than a PICC. A randomized controlled study and formal cost-effective analysis would be needed to determine the net benefit of routine use of CICC over PICC in this population.
Abbreviations
- AL:
-
Acute leukemia
- CICC:
-
Centrally inserted central venous catheter
- CRT:
-
Catheter-related thrombosis
- CVC:
-
Central venous catheter
- DVT:
-
Deep vein thrombosis
- HRT:
-
Hormonal replacement therapy
- HSCT:
-
Hematopoietic stem cell transplant
- OCP:
-
Oral contraceptive pills
- PE:
-
Pulmonary embolism
- PICC:
-
Peripherally inserted central venous catheter
- sHR:
-
Subhazard ratio
- VTE:
-
Venous thromboembolism
References
Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, Becker RC, Goldberg RJ (2006) The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 21(7):722–727. doi:10.1111/j.1525-1497.2006.00458.x
Spencer FA, Emery C, Joffe SW, Pacifico L, Lessard D, Reed G, Gore JM, Goldberg RJ (2009) Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis 28(4):401–409. doi:10.1007/s11239-009-0378-3
Streiff MB (2013) Association between cancer types, cancer treatments, and venous thromboembolism in medical oncology patients. Clin Adv in Hematol Oncol 11(6):349–357
Smith JR, Friedell ML, Cheatham ML, Martin SP, Cohen MJ, Horowitz JD (1998) Peripherally inserted central catheters revisited. Am J Surg 176(2):208–211
Geerts W (2014) Central venous catheter-related thrombosis. Hemato Educ Program Am Soc Hematol Am Soc Hematol Educ Program 2014(1):306–311. doi:10.1182/asheducation-2014.1.306
Catalano O, Nunziata A, de Lutio di Castelguidone E, d’Errico AG (2011) Thrombosis and cancer: spectrum of multidetector CT findings in oncologic patients with thromboembolic disease. A pictorial review. Acta Radiol (Stockholm, Sweden : 1987) 52(7):730–737. doi:10.1258/ar.2011.100513
Mollee P, Jones M, Stackelroth J, van Kuilenburg R, Joubert W, Faoagali J, Looke D, Harper J, Clements A (2011) Catheter-associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect 78(1):26–30. doi:10.1016/j.jhin.2011.01.018
Saber W, Moua T, Williams EC, Verso M, Agnelli G, Couban S, Young A, De Cicco M, Biffi R, van Rooden CJ, Huisman MV, Fagnani D, Cimminiello C, Moia M, Magagnoli M, Povoski SP, Malak SF, Lee AY (2011) Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J Thromb Haemost 9(2):312–319. doi:10.1111/j.1538-7836.2010.04126.x
Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, Flanders SA (2013) Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 382(9889):311–325. doi:10.1016/s0140-6736(13)60592-9
Liu Y, Gao Y, Wei L, Chen W, Ma X, Song L (2015) Peripherally inserted central catheter thrombosis incidence and risk factors in cancer patients: a double-center prospective investigation. Ther Clin Risk Manag 11:153–160. doi:10.2147/tcrm.s73379
Cortelezzi A, Moia M, Falanga A, Pogliani EM, Agnelli G, Bonizzoni E, Gussoni G, Barbui T, Mannucci PM (2005) Incidence of thrombotic complications in patients with haematological malignancies with central venous catheters: a prospective multicentre study. Br J Haematol 129(6):811–817. doi:10.1111/j.1365-2141.2005.05529.x
Worth LJ, Seymour JF, Slavin MA (2009) Infective and thrombotic complications of central venous catheters in patients with hematological malignancy: prospective evaluation of nontunneled devices. Support Care Cancer 17(7):811–818. doi:10.1007/s00520-008-0561-7
Tran H, Arellano M, Chamsuddin A, Flowers C, Heffner LT, Langston A, Lechowicz MJ, Tindol A, Waller E, Winton EF, Khoury HJ (2010) Deep venous thromboses in patients with hematological malignancies after peripherally inserted central venous catheters. Leuk Lymphoma 51(8):1473–1477. doi:10.3109/10428194.2010.481065
Sriskandarajah P, Webb K, Chisholm D, Raobaikady R, Davis K, Pepper N, Ethell ME, Potter MN, Shaw BE (2015) Retrospective cohort analysis comparing the incidence of deep vein thromboses between peripherally-inserted and long-term skin tunneled venous catheters in hemato-oncology patients. Thromb J 13:21. doi:10.1186/s12959-015-0052-2
Mohren M, Markmann I, Jentsch-Ullrich K, Koenigsmann M, Lutze G, Franke A (2006) Increased risk of venous thromboembolism in patients with acute leukaemia. Br J Cancer 94(2):200–202. doi:10.1038/sj.bjc.6602945
Kopolovic I, Lee AY, Wu C (2015) Management and outcomes of cancer-associated venous thromboembolism in patients with concomitant thrombocytopenia: a retrospective cohort study. Ann Hematol 94(2):329–336. doi:10.1007/s00277-014-2198-6
Fine J, Gray R (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94(446):496–509
Campigotto F, Neuberg D, Zwicker JI (2012) Accounting for death as a competing risk in cancer-associated thrombosis studies. Thromb Res 129(Suppl 1):S85–S87. doi:10.1016/s0049-3848(12)70023-3
StataCorp (2013) Stata Statistical Software, vol Release 13. StataCorp LP, College Station
Kim HJ, Yun J, Kim HJ, Kim KH, Kim SH, Lee SC, Bae SB, Kim CK, Lee NS, Lee KT, Park SK, Won JH, Park HS, Hong DS (2010) Safety and effectiveness of central venous catheterization in patients with cancer: prospective observational study. J Korean Med Sci 25(12):1748–1753. doi:10.3346/jkms.2010.25.12.1748
Ratcliffe M, Broadfoot C, Davidson M, Kelly KF, Greaves M (1999) Thrombosis, markers of thrombotic risk, indwelling central venous catheters and antithrombotic prophylaxis using low-dose warfarin in subjects with malignant disease. Clin Lab Haematol 21(5):353–357
Cortelezzia A, Fracchiolla NS, Maisonneuve P, Moia M, Luchesini C, Ranzi ML, Monni P, Pasquini MC, Lambertenghi-Deliliers G (2003) Central venous catheter-related complications in patients with hematological malignancies: a retrospective analysis of risk factors and prophylactic measures. Leuk Lymphoma 44(9):1495–1501. doi:10.3109/10428190309178770
Abdelkefi A, Torjman L, Ladeb S, Othman TB, Achour W, Lakhal A, Hsairi M, Kammoun L, Hassen AB, Abdeladhim AB (2005) Randomized trial of prevention of catheter-related bloodstream infection by continuous infusion of low-dose unfractionated heparin in patients with hematologic and oncologic disease. J Clin Oncol 23(31):7864–7870. doi:10.1200/jco.2004.00.9787
Lordick F, Hentrich M, Decker T, Hennig M, Pohlmann H, Hartenstein R, Peschel C (2003) Ultrasound screening for internal jugular vein thrombosis aids the detection of central venous catheter-related infections in patients with haemato-oncological diseases: a prospective observational study. Br J Haematol 120(6):1073–1078
van Rooden CJ, Schippers EF, Barge RM, Rosendaal FR, Guiot HF, van der Meer FJ, Meinders AE, Huisman MV (2005) Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: a prospective study. J Clin Oncol 23(12):2655–2660. doi:10.1200/jco.2005.05.002
Yardim H, Erkoc R, Soyoral YU, Begenik H, Avcu S (2012) Assessment of internal jugular vein thrombosis due to central venous catheter in hemodialysis patients: a retrospective and prospective serial evaluation with ultrasonography. Clin Appl Thromb Hemost 18(6):662–665. doi:10.1177/1076029611432739
Aw A, Carrier M, Koczerginski J, McDiarmid S, Tay J (2012) Incidence and predictive factors of symptomatic thrombosis related to peripherally inserted central catheters in chemotherapy patients. Thromb Res 130(3):323–326. doi:10.1016/j.thromres.2012.02.048
Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, Rosendaal FR (2004) Estrogen plus progestin and risk of venous thrombosis. JAMA 292(13):1573–1580. doi:10.1001/jama.292.13.1573
ACOG Practice Bulletin No. 138 (2013) Inherited thrombophilias in pregnancy. Obstet Gynecol 122(3):706–717. doi:10.1097/01.AOG.0000433981.36184.4e
Grainge MJ, West J, Card TR (2010) Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 375(9715):657–663. doi:10.1016/s0140-6736(09)61963-2
Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH (1996) The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 125(1):1–7
Samama MM (2000) An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med 160(22):3415–3420
Allman-Farinelli MA (2011) Obesity and venous thrombosis: a review. Semin Thromb Hemost 37(8):903–907. doi:10.1055/s-0031-1297369
Freeman AL, Pendleton RC, Rondina MT (2010) Prevention of venous thromboembolism in obesity. Expert Rev Cardiovasc Ther 8(12):1711–1721. doi:10.1586/erc.10.160
Elishoov H, Or R, Strauss N, Engelhard D (1998) Nosocomial colonization, septicemia, and Hickman/Broviac catheter-related infections in bone marrow transplant recipients. A 5-year prospective study. Medicine 77(2):83–101
Yap YS, Karapetis C, Lerose S, Iyer S, Koczwara B (2006) Reducing the risk of peripherally inserted central catheter line complications in the oncology setting. Eur J Cancer Care 15(4):342–347. doi:10.1111/j.1365-2354.2006.00664.x
Boersma RS, Jie KS, Verbon A, van Pampus EC, Schouten HC (2008) Thrombotic and infectious complications of central venous catheters in patients with hematological malignancies. Ann Oncol 19(3):433–442. doi:10.1093/annonc/mdm350
de Marie S (1993) Diseases and drug-related interventions affecting host defence. Eur J Clin Microbiol Infect Dis 12(Suppl 1):S36–S41
Authors’ contribution
M. Refaei and C. Wu developed the research protocol. M. Refaei and B. Fernandes performed primary data collection. M. Refaei prepared the manuscript. A. Pokhrel performed statistical analysis. Manuscript was reviewed and approved for publication by J. Brandwein, M. D. Goodyear, and C. Wu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approvals from both cities were obtained (Edmonton Pro00051738; Calgary REB14-1916), in keeping with the Canadian Tri-Council Policy statement on ethical conduct for research.
Conflict of interest
C. Wu is a member on advisory board with honoraria from Leo Pharma and Pfizer. M.D. Goodyear has received research grants from Bayer and Pfizer. The rest of the authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table S1-Table S3
Incidence of Catheter-related Thrombosis in Acute Leukemia patients: a comparative, retrospective study of the safety of Peripherally-Inserted vs. Centrally-Inserted Central Venous Catheters. (DOCX 84 kb)
Rights and permissions
About this article
Cite this article
Refaei, M., Fernandes, B., Brandwein, J. et al. Incidence of catheter-related thrombosis in acute leukemia patients: a comparative, retrospective study of the safety of peripherally inserted vs. centrally inserted central venous catheters. Ann Hematol 95, 2057–2064 (2016). https://doi.org/10.1007/s00277-016-2798-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2798-4