Abstract
Myeloproliferative neoplasms are characterized by a common stem cell-derived clonal proliferation, but are phenotypically diverse. JAK2 is mutated (V617F) in more than 90 % of patients with polycythemia vera (PV) and approximately 60 % of patients with essential thrombocythemia (ET) or primary myelofibrosis (PMF). Pulmonary arterial hypertension (PAH) is a major complication of several hematological disorders. Chronic myeloproliferative disorders associated with PAH have been included in group five for which the etiology is unclear and/or multifactorial. The aim of this study is to screen Egyptian Philadelphia negative JAK2 positive myeloproliferative neoplasm patients for the presence of PAH and its correlation with JAK2 allele burden. We also made a review for correlation of JAK2 allele with hematological parameters comparing our results to others. We enrolled 60 patients with Philadelphia negative myeloproliferative neoplasms. All patients enrolled in the study were subjected to laboratory and imaging workup in the form of CBC, liver, kidney profile, bone marrow examination, abdominal ultrasonography, and transthoracic echocardiography. Our results revealed that 7 patients out of 60 (11.67 %) had pulmonary arterial hypertension, 3 patients with PMF, 2 patients with PRV, and 2 patients with ET, and its correlation with JAK2 allele burden was not statistically significant. Correlation analysis between JAK2 V617F allele burden and other parameters revealed: statistical significant correlation with age, HB, HCT, PLT, UA, LDH, and splenic diameter but insignificant correlation with WBCs and PAH. Pulmonary arterial hypertension prevalence in our study was 11.67 % and no significant correlation with JAK 2 allele burden. Our study is the largest one up to our knowledge that studies the association between its prevalence and JAK2 burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myeloproliferative neoplasms are characterized by a common stem cell-derived clonal proliferation, but are phenotypically diverse due to differences in genetic rearrangements or mutations, and now it has been clearly understood with the discovery that the protein tyrosine kinase JAK2 is mutated (V617F) in more than 90 % of patients with polycythemia vera (PV) and approximately 60 % of patients with essential thrombocythemia (ET) or primary myelofibrosis (PMF) [1]. The mechanism(s) behind this one allele multiple phenotypes phenomenon has not been fully elucidated. The issue is further confounded by the presence of marked variation in JAK2 V617F allele burden among mutation positive patients [2].
Pulmonary hypertension (PAH) is a major complication of several hematological disorders. The incidence of primary PAH in myeloproliferative neoplasm (MPN), once secondary causes are excluded, seems quite high. As established mainly by transthoracic echocardiography (TTE), the PAH prevalence was 44 % in essential thrombocythemia (ET) [3], 22 % in polycythemia rubra vera (PRV) [4], 37 % in primary myelofibrosis (PMF) [5], and 25 % in chronic myeloid leukemia (CML) [6], with a global prevalence of 38 %. Increased pulmonary artery pressure in patients with MPN may result from various mechanisms including anemia, and a hypermetabolic state with high cardiac output and left ventricular dysfunction [7]. The possible association between PAH and Philadelphia negative MPN has been suggested by several case reports and small case series [8]. However, the prevalence and incidence of PH in the context of MPN may be underestimated since the clinical signs of disease appear at an advanced stage of the disease and, in some cases, the diagnosis of MPN is difficult to establish in the context of chronic hypoxia [7].
Also, several studies concerned about the relation between hemogram and the presence of JAK2 with controversial results, but only few studies which correlate the JAK2 allele’s burden with the different parameters.
This prompted us to perform a more extensive study enrolling a higher number of patients from a single center; 60 patients with Philadelphia negative JAK2 positive myeloproliferative neoplasm and the correlation with JAK2 V617F allele burden.
Patients and methods
The current study was conducted over 20 months starting from May 2013 after approval of the protocol by Research Ethical Committee. In the present study, we enrolled 60 adult Philadelphia negative JAK2 positive myeloproliferative patients from the Hematology Clinic in Kasr Al Aini Hospital; 29 PRV (48.3 %), 18 ET (30 %), and 13 PMF (21.7 %). Patients with known pulmonary hypertension due to specific heart disease were excluded. All patients enrolled in the study after obtaining a written consent. All the patients in the study were subjected to full medical history, complete clinical examination, and laboratory investigations including CBC, ALT, AST, urea, creatinine, uric acid, and LDH. Abdominal ultrasound to assess splenic diameter and transthoracic echocardiography to estimate the tricuspid regurgitation velocity (TRV) (m/s) as well as to assess the probability of pulmonary hypertension were performed as advised by 2015 ESC/ERS Guidelines [9] for the diagnosis of pulmonary hypertension (Tables 1 and 2). The JAK2 allele burden was assessed by JAK-2 MutaQuant kit (IPSOGEN Cancer Profile) in human genomic DNA using ABI PRISM 7300 Sequence Detection System.
Statistics
All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows. Data were statistically described in terms of mean ± standard deviation (±SD), and range. Comparison of non-parametric variables was done using Mann–Whitney U test for independent samples when comparing two groups and Kruskal–Wallis test when comparing more than two groups. Comparison of parametric values between two groups was done using t test, ANOVA, and post hoc study when comparing more than two groups. For comparing categorical data, Chi-square (χ 2) test was performed. Correlation analysis using Spearman’s test P values were significant if less than 0.05.
Results
We enrolled 60 Philadelphia negative JAK2 positive MPNs patients; 33 females (55 %) and 27 males (45 %); with the following subtypes 29 PRV (48.3 %), 18 ET (30 %), and 13 PMF (21.7 %).
Table 3 demonstrates all demographic and laboratory results.
Echocardiographic data revealed that 53 patients (88.3 %) had low probability of pulmonary arterial hypertension (PAH); 27 PRV patients (45 %), 16 ET patients (26.6 %), and 10 PMF patients (16.7 %) while the remaining 7 patients (11.6 %) had intermediate probability of PAH; 2 cases of PRV (3.3 %), 2 cases of ET (3.3 %), and 3 cases of PMF (5 %).
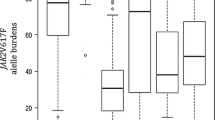
Correlation analysis between JAK2 V617F allele burden and other parameters as shown in Table 4, revealing statistical significant correlation with age, HB, HCT, PLT, UA, LDH, and splenic diameter but insignificant correlation with WBCs and PAH.
Comparisons of the two groups of PAH according to different parameters as shown in Table 5, revealing statistical significant difference in WBCs count only.
Comparison between the three groups of myeloproliferative disorders according to PAH revealed insignificant difference p = 0.11.
Discussion
Pulmonary arterial hypertension (PAH) is well known in myeloproliferative neoplasms (MPN) [10]. MPN with PAH have been included in group 5 of the clinical classification for PAH. Compared to previous studies of the same concern, the current study has unique feature as we did not only studied the prevalence of PAH but also the correlation between PAH and JAK2 allele’s burden in Egyptian patients with Philadelphia negative JAK2 positive MPN.
Right ventricular systolic pressure (RVSP) evaluation by transthoracic echo (TTE) as a utility for diagnosing PAH is good being noninvasive methods and excluding cardiac causes of PAH, but on the other hand PAH may be overestimated by this utility [7], and it was advised in 2015 that PAH probability is to be assessed by tricuspid regurgitation velocity (TRV) utility [9] as done in our study to eliminate a major probability of false positive PAH as possible, also the accurate anamnesis of patient cardiovascular history together with carful elimination of left ventricular dysfunction [11] may eliminate a major proportion of false positive PAH diagnosis.
Among our studied 60 Philadelphia negative JAK2 positive MPN patients, we found 7 patients (11.67 %) with intermediate pulmonary arterial hypertension (PAH). Reisener SA et al. found 4 (13 %) out of 30 MPN patients with PAH unrelated to valvular disease [12]. Garypidou V et al. studied 24 MPN patients, the majority of them (14 out of 24) were ET and found that 10 (41.7 %) patients had PAH [10]. Gupta R et al. described also PAH in 12 (48 %) out of 25 patients with MPNs [13] Altintas A et al. found PAH in 22 (47.8 %) patients out of 46 ET patients [3]. A larger study by Chebrek S et al. who evaluated 68 patients with MPNs (27 ET, 15 PMF, and 26 PV) found lower prevalence of PAH. Only five patients (7 %) were found to have increased pulmonary artery pressure. However, this work did not study the correlation with JAK2 allele burden [14].
The variability in prevalence of PAH in previously mentioned studies and our study make our main concern to investigate the relation between the JAK2 allele’s burden and the degree or the severity of PAH as well as the clinical relevance in our studied MPN patients.
In our work, we did not find any correlation between the JAK2 allele’s burden and the presence of PAH, and this correlation was not addressed till now by any other studies.
In our study, none of the studied parameters including age, sex, splenic diameter, hemoglobin levels, or platelet count was predictive of the presence of PAH, except for WBCs that was significantly different among two groups of PAH, and this finding was in agreement with both Garypidou V et al. [10] and Gupta R et al. [13], and on the other hand is not consistent with Altintas A et al. [3] and Dingli D et al. [15], who found significant correlation between higher platelets counts and PAH.
Several studies concerned about the relation between hemogram and JAK2 status (absence or presence) with controversial results as shown in Table 6, but only few studies correlated the JAK2 allele’s burden with the different parameters.
In our study, we found that there is a positive statistically significant correlation between the JAK2 allele’s burden and the hemoglobin level, splenic diameter and LDH, and negative statistically significant correlation with the platelet count. Zohu et al. found positive significant correlation for JAK2 allele’s burden with increasing HB but not for platelet count in 222 mutated JAK2 V617F patients [16]. Zahang et al. found that a higher JAK2 V617F load correlated significantly with higher HB% level in studied PV patients [17]. Kittur et al. found that JAK2 allele’s burden correlated significantly with lower platelet count, palpable splenomegaly at diagnosis, and venous thrombosis occurring after diagnosis in 69 ET patients [18]. Larsen TS et al. found that the JAK2 allele’s burden correlated significantly with higher levels of LDH and as well as lower platelet counts in 165 Philadelphia negative MPN patients [19]. Silver et al. found that there is a correlation between the splenic size and the JAK2 allele’s burden [20].
On the other hand, studies by Larsen et al. [19], Kittur et al. [18], Zohu et al. [16], and Silver RT et al. [20] determined that higher JAK2 allele’s burden correlated with a higher white blood cell count which is different from our finding that there is no significant correlation between JAK2 allele’s burden and white cell count in our studied patients.
These differences may be due to the differences among studies regarding the demographic features of studied population, whether studied population on treatment or not, different modalities of treatment, and the number of studied groups. We believe that meta-analysis of similar studies all together is important to know the exact incidence and prevalence of PAH in those patients and its correlation with other parameters.
Referances
Vannucchi AM, Guglielmelli P, Tefferi A (2009) Advances in understanding and management of myeloproliferative neoplasms. CA Cancer J Clin 59:171–191
Kim HR, Choi HJ, Kim YK et al (2013) Allelic expression imbalance of JAK2 V617F mutation in BCR-ABL negative myeloproliferative neoplasms. PLoS One 8(1):e52518
Altintas A, Karahan Z, Pasa S et al (2007) Pulmonary hypertension in patients with essential thrombocythemia and reactive thrombocytosis. Leuk Lymphoma 48:1981–1987
Kadikoylu G, Onbasili A, Tekten T et al (2004) Functional and morphological cardiac changes in myeloproliferative disorders (clinical study). Int J Cardiol 97:213–220
Cortelezzi A, Gritti G, Del Papa N et al (2008) Pulmonary arterial hypertension in primary myelofibrosis is common and associated with an altered angiogenic status. Leukemia 22:646–649
Roach EC, Park MM, Tang WH et al (2015) Impaired right ventricular-pulmonary vascular function in myeloproliferative neoplasms. J Heart Lung Transplant 34(3):390–394
Adir Y, Humbert M (2010) Pulmonary hypertension in patients with chronic myeloproliferative disorders. Eur Respir J 35:1396–1406
Barbui T, Barosi G, Birgegard G et al (2011) Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European leukemia Net. J Clin Oncol 29(6):761–770
Galiè N, Humbert M, Vachiery JL et al (2016) 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J 37(1):67–119
Garypidou V, Vakalopoulou S, Dimitriadis D et al (2004) Incidence of pulmonary hypertension in patients with chronic myeloproliferative disorders. Hoematologica 89:245–246
Janda S, Shahidi N, Gin K, Swiston J (2011) Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart 97(8):612–622
Reisner SA, Rinkevich D, Markiewicz W et al (1992) Cardiac involvement in patients with myeloproliferative disorders. Am J Med 93:498–504
Gupta R, Perumandla S, Patsiornik Y et al (2006) Incidence of pulmonary hypertension in patients with chronic myeloproliferative disorders. J Natl Med Assoc 98:1779–1782
Chebrek S, Aïssi K, Francès Y et al (2014) Pulmonary hypertension in patients with chronic myeloproliferative neoplasm. Leuk Lymphoma 55:223–225
Dingli D, Utz JP, Krowka MJ et al (2001) Unexplained pulmonary hypertension in chronic myeloproliferative disorders. Chest 120:801–808
Zhou J, Ye Y, Zeng S et al (2013) Impact of JAK2 V617F mutation on hemogram variation in patients with Non-reactive elevated platelet counts. PLoS One 8(2):e57856
Zhang Y, Li L, Nie L et al (2008) Clinical study on relationship between JAK2 V617F mutation and chronic myeloproliferative disorders. Zhonghua Xue Ye Xue Za Zhi 29(2):105–109
Kittur J, Knudson RA, Lasho TL et al (2007) Clinical correlates of JAK2V617F allele burden in essential thrombocythemia. Cancer 109:2279–2284
Larsen TS, Pallisgaard N, Møller MB, Hasselbalch HC (2007) The JAK2 V617F allele burden in essential thrombocythemia, polycythemia vera and primary myelofibrosis—impact on disease phenotype. Eur J Haematol 79(6):508–515
Silver RT, Vandris K, Wang YL et al (2011) JAK2 (V617F) allele burden in polycythemia vera correlates with grade of myelofibrosis, but is not substantially affected by therapy. Leuk Res 35:177
Sultan S, Irfan SM (2015) Acquired JAK-2 V617F mutational analysis in Pakistani patients with essential thrombocythemia. Asian Pac J Cancer Prev 16(16):7327–7330
Vytrva N, Stacher E, Regitnig P et al (2014) Megakaryocytic morphology and clinical parameters in essential thrombocythemia, polycythemia vera, and primary myelofibrosis with and without JAK2 V617F. Arch Pathol Lab Med 138:1203
Duletic AN, Dekanic A, Hadzisejdic I et al (2012) JAK2-V617F mutation is associated with clinical and laboratory features of myeloproliferative NeoplasmsColl. Antropol 3:859–865
Chao HY, Fan Z, Zhang R, et al (2009) Detection and clinical significance of JAK2 mutation in 412 patients with chronic myeloproliferative neoplasms. Zhonghua Zhong Liu Za Zhi 31(7):510–4
Ilhan G, Karakus S, Sahin FI (2012) JAK 2V617F mutation: frequency and relation to clinical and laboratory features of BCR-ABL negative myeloproliferative diseases. Int J Hematol Oncol 2:77–84
Pemmaraju N, Moliterno AR, Williams DM et al (2007) The quantitative JAK2 V617F neutrophil allele burden does not correlate with thrombotic risk in essential thrombocytosis. Leukemia 21:2210–2212
Barosi G, Bergamaschi G, Marchetti M et al (2007) JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood 110:4030–4036
Campbell PJ, Scott LM, Buck G et al (2005) Definition of subtypes of essential thrombocythemia and relation to polycythemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 366(9501):1945–1953
Garcia C, de Carranza ST, Campos CB et al (2007) Correlation between LDH levels and mutational status of JAK2 gene in essential thrombocythemia. Haematologica 92(suppl2):547
Acknowledgment
Special thanks to all workers and patients of hematolgy clinic. Mervat Mattar designed the research study and revised the results and the paper. Mohammed Abdel Kader selected the patients and participated in collecting references for paper. Noha Ali did the ECHO to the patients. Doaa El Demerdash aided in writing the paper. Noha El Husseiny performed statistics and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mattar, M.M., Morad, M.A.K., El Husseiny, N.M. et al. Correlation between JAK2 allele burden and pulmonary arterial hypertension and hematological parameters in Philadelphia negative JAK2 positive myeloproliferative neoplasms. An Egyptian experience. Ann Hematol 95, 1611–1616 (2016). https://doi.org/10.1007/s00277-016-2765-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2765-0