Abstract
Background: MiR-126 is likely to be closely associated with the threatening disease deep venous thrombosis (DVT). Aim: This study aims to investigate the influence of aberrantly expressed miR-126 on vascular endothelial cell (VEC) apoptosis during DVT and explore how miR-126 functions in it. Methods: MiR-126 inhibition and overexpression in vivo were respectively performed with antagomir and agomir of miR-126. Using a rat traumatic femoral DVT model, VEC apoptosis and miR-126 expression were detected by TUNEL assay and qRT-PCR before thrombogenesis and at different time phases of thrombogenesis. Protein levels of MMPs, Akt, Bcl-2, Bad, and caspase-9 in vascular tissue were measured by western blotting. In vitro, miR-126 interference, and overexpression were performed on human umbilical vein endothelial cells (HUVECs) using miR-126 inhibitor and mimics. After HUVECs were pretreated with CoCl2, cell apoptosis was analyzed using flow cytometry, and RNA/protein levels of miR-126, PIK3R2, PTEN, and phosphorylated Akts were measured with qRT-PC/western blotting. Results: The apoptosis of VECs was increased by miR-126 inhibition and obviously rescued by miR-126 overexpression. PI3K/Akt signal transduction was suppressed by miR-126 inhibition and evidently enhanced by miR-126 overexpression. Consistent with these findings, the downstream proteins (Bcl-2, Bad, and cleaved caspase-9) in PI3K/Akt pathway and the MMPs were remarkably changed by inhibition or overexpression of miR-126. In vitro experiments also showed that PI3K/Akt signaling was strengthened when miR-126 expression was upregulated or inhibited when miR-126 was knockdown. Conclusion: Overexpressed miR-126 inhibits apoptosis of VECs and DVT through targeting the anti-apoptotic pathway PI3K/Akt via PIK3R2. General significance: These findings may provide a new target for the therapy of DVT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep venous thrombosis (DVT), an abnormal blood coagulation in vena profunda, has great perniciousness and is frequently seen in surgery especially in orthopedics [1]. In China, there are 10 million DVT cases that are diagnosed every year [2]. It has been widely believed that damaged vascular walls, blood flow change, and abnormal blood composition are three major factors for thrombogenesis. The damage to vascular walls mainly refers to the injury of vascular endothelial cells (VECs). Once the injury and/or apoptosis of VECs occurs, not only antithrombotic function will be lost, but also it will become an initiating factor of thrombus, and will even increase phosphatidylserine expression, deprive the asymmetry of membrane phospholipids, and delete anticoagulant membrane composition, and as a result, thrombosis will be remarkably promoted [3, 4]. The occurrence and regulation of apoptosis are involved in an extremely complicated system that is still unrevealed, where varieties of signal pathways exist. In our previous study, various signal pathways like PI3K/AKT, Wnt, MAPK, and Toll-like receptor signaling were found to be significantly changed after DVT, and the anti-apoptotic signal might affect the biological state of thrombus through regulating VECs and finally caused DVT [5].
MicroRNAs (miRNAs) are endogenous small-noncoding single-stranded molecules consisting of about 22 nucleotides. They suppress the translation of target genes or degrade the RNA transcripts of target genes to regulate gene expression via binding to 3′ untranslated region (3′UTR) of target genes. MiRNAs extensively participate in the regulation of physiology and pathology of various types of cells or tissues in the body, and they are closely related to the regulation of cell differentiation, proliferation, and apoptosis and the processes like the development and metabolism of tissue and organ [6]. Some overexpressed specific miRNAs in VECs are recently found to play important roles in physiological and pathological processes of blood vessels through regulating the expressions of some endothelial cell-related genes, such as endothelin-1 (ET-1) and NO [7]. MiR-126 is an miRNA that is very closely associated with VECs and vascular function, and its gene is located in the intron region between the 7th and 8th exons of epidermal-gowth factor-like domain 7 (EGFL7); hence, the consistency with EGFL7 that miR-126 has VEC specificity like EGFL7 [8, 9].
It was reported that miR-126 regulated and controlled the expressions of quite a few of genes, such as Spred-1, SDF-1, VCAM-1, HoxA9, v-Crk, EGFL-7, and VEGF, and therefore, it adjusted the vascular development, neovascularization, and vascular inflammation, etc. [8, 10, 11]. Further studies demonstrated that miR-126 was able to promote PI3K expression through directly targeting its negative regulator PIK3R2 (phosphoinositide-3-kinase regulatory subunit 2, also called p85β) [12]. Our previous studies also showed that the expressions of PI3K and Akt were decreasing with the development of DVT, where it should be noted that PI3K/Akt is one of the anti-apoptosis pathways in VECs [5, 13, 14]. Both microRNA target prediction and the studies that is already done suggest that PIK3R2, which can effectively inhibit PI3K activation, is one of the target genes of miR-126 [15]. Based on these findings, we speculated that miR-126 probably regulated the apoptosis of VECs during DVT through targeting PI3K/Akt signaling.
To explore how miR-126 functions in DVT, the influence of differentially expressed miR-126 on VEC apoptosis was observed and the regulating function of miR-126 was investigated in this study, using both DVT rats and in vitro HUVECs with oxidative damage.
Materials and methods
Experimental animals and cell line
Sprague Dawley rats (250 ± 20 g) were obtained from Shanghai Laboratory Animal Center (Shanghai, China) and were fed in a specific pathogen-free animal house. And all animal-related experiments in this study were approved by Animal Ethical Committee of Kunming Medical University, and the corresponding procedures performed here were conducted strictly according to the official guidance from Instructive Notions with Respect to Caring for Laboratory Animals published in 2006 by Science and Technology Department of China. HUVECs were obtained from ScienCell Research Laboratories (San Diego, CA, USA) and cultured in endothelial cell medium supplemented with 5 % FBS, 1 % penicillin/streptomycin solution (Life Technologies, Inc., Paisley, UK), and 10 % endothelial cell growth supplement (ECGS; Sigma, St Louis, MO) [16].
Rat traumatic limb DVT model and grouping
DVT model
Sprague Dawley rats (36 male and 36 female), weighing 250 ± 20 g, were selected for this study. The model was established as previously reported [5]. In brief, a self-made quantitative hit device, which provided a ~5 Joule instant hit energy, was used to make a traumatic DVT at a fixed point in bilateral thighs of the rats that had been anesthetized with 30 mg/kg pentobarbital sodium. Rats were placed in the prone position and the lateral side of their proximal thigh was hit once in the range from trochanter major to 1 cm bellow. After femoral fracture was made, stabilization with plaster spica of the hip was performed. MiR-126 determination, TUNEL assay for cell apoptosis of vascular endothelial cell in femoral, and determination of PI3K/Akt-related gene expressions were respectively performed at 2.5 (before thrombosis) and 25 h (after thrombogenesis) post trauma.
Grouping
The rats were randomly divided into seven groups, which were designated as normal, model 2.5 h, model 25 h, 2.5 h/inhibition, 25 h/inhibition, 2.5 h/overexpression, 25 h/overexpression, where the 2.5 and 25 h, respectively, meant 2.5 and 25 h post-trauma, and inhibition and overexpression were, respectively, short for miR-126 inhibition and miR-126 overexpression. MiR-126 inhibition and overexpression in vivo were accordingly performed through injection at both proximal thighs with antagomir or agomir of miR-126, which are a kind of chemically modified microRNA antagonists or simulants.
MiR-126 agomir and antagomir transfection
For overexpression and inhibition of miR-126, agomir-126 and antagomir-126 (Ribobio, Guangzhou, China) were respectively used to inject locally at proximal thighs. Based on the manufacturer’s protocols, transfection compounds were prepared at the suggested optimal concentration (400 pmol agomir-126 or antagomir-126 in 20 ml transfection compounds). The rats in normal group were injected with 20 ml saline. In miR-126 overexpression or inhibition groups, rats were injected with 20 ml of the above-mentioned transfection compounds containing 400 pmol agomir-126 or antagomir-126 everyday for continuous 4 days. After the miR-126 overexpression or inhibition in the rats was confirmed by qRT-PCR using one random rat in each experimental group, all of the left rats were anesthetized and executed. Subsequently, about 3 cm of the femoral vein and the main branches were extracted from bilateral thighs under aseptic conditions. If there was thrombus in the blood vessel, it would be removed, following which, the vascular wall was washed and the blood in the vessel lumen was absterged for qRT-PCR, TUNEL assay, and western blotting. All of the samples were immediately put into cryogenic vials and then preserved in liquid nitrogen. The in vivo transfection efficiency of agomir-126 and antagomir-126 was determined by detecting the expression of miR-126 in femoral veins.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
Serial 6-μm-paraffin sections were dried at 62 °C in an air oven for 2 h, after which, the sections were dewaxed with xylene, hydrated with ethyl alcohol, and washed thrice with PBS for 3 min (each time). The samples were incubated with proteinase K at room temperature for 30 min, and then washed again with PBS as ditto. In order to inhibit endogenous peroxidase, H2O2 (3 %) was added to the samples and incubation at room temperature was performed for 20 min. After washed with PBS three times, the sections were incubated in equilibration buffer at room temperature for 20 min. Bibulous paper was used to absorb most of the equilibration buffer, and TdT (terminal deoxynucleotidyl transferase) incubation buffer was added for the following incubation at 37 °C for 1 h. The sections were washed with PBS, following which, serum was added and incubation at 37 °C lasted for 30 min. Cell nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; Biohao Biotec co, Ltd., Shanghai, China) for 20 min. After PBS washing, the reagent (Beyotime Biological Technology Ltd., Shanghai, China) that prevents fluorescence quenching was added. The sections was mounted and reserved at −20 °C. Images of the fluorescent immunohistochemistry were photographed at ×200 magnification under a fluorescence microscope (XDY-1, Microscopes Inc., St. Louis, MI, USA). In each photo, the stained cells were manually counted using AxioVision 4.2 software (Carl Zeiss, Thornwood, NY, USA). The cells were counted by three researchers who had no knowledge of the treatment status of each rat.
Quantitative RT-PCR (qRT-PCR)
Total RNA was isolated using an RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the protocols. U6 was used as an internal reference for miR-126 detection. The cDNA was synthesized from 1 μg total RNA, then it was used as templates for PCR amplification: denaturation at 95 °C for 5 min, 95 °C for 10 s, 60 °C for 20 s, 72 °C for 20 s and 78 °C for 20 s, 40 circulations totally.
Referring to the literature [17], β-actin was used as an internal reference for detecting mRNAs of MMP-1, MMP-13, and TIMP-1. A total RNA sample (1 μg) was reverse transcribed, and amplified by a PCR amplifier (qTOWER 2.2, Analytik Jena AG, Germany) with conditions as follows: denaturation at 94 °C, extension at 72 °C, and annealing at 52 °C for 20, 25, and 30 cycles. The primers were provided in the supplementary materials.
Data were presented as fold changes relative to either β-actin for MMP-1, MMP-13, and TIMP-1 or U6 for miR-126 based on calculations of 2−ΔΔCt. All qRT-PCR experiments were performed in triplicate, including no-template controls.
Western blot analysis
Western blotting was performed to measure the protein levels of MMP-1, MMP-13, TIMP-1, pS473Akt, pT308Akt, Akt, Bcl-2, Bad, procaspase-9, and cleaved caspase-9. Cell lysate was prepared using RIPA Buffer (50 mM Tris–HCl, pH 8.0/150 mM NaCl/1 % (vol/vol) Nonidet P-40/0.5 % (wt/vol) sodium desoxycholate/0.1 % (wt/vol) SDS) with a protease inhibitor Na orthovanadate (0.1 mM), and the protein concentrations were determined using BCA protein assay kit (Pierce, Rockford, IL, USA). The protein (20 mg) was loaded onto a 10 % SDS–PAGE gel then transferred to nitrocellulose and incubated with rat monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) against the above-mentioned proteins at 4 °C overnight in 1 % non-fat dry milk. Then incubation with HRP-conjugated secondary anti-rat antibodies was performed. Protein was normalized with β-actin. Blots were then developed using ECL Substrate (Pierce, Rockford, IL, USA) following manufacturer’s instructions. Densitometric analyses were performed using the ImageJ software.
MiR-126 overexpression and knockdown in HUVECs
To investigate the role of miR-126 in HUVECs, chemosynthetic miR-126 inhibitor and miR-126 mimics were respectively used to downregulate and upregulate miR-126 expression. The RNA oligos were synthesized by GenePharma (Shanghai, China) and their sequences were as follows:
-
Has-miR-126 inhibitor: 5′-CGCAUUAUUACUCACGGUACGA-3′
-
Inhibitor negative contro1: 5′-CAGUACUUUUGUGUAGUACAA-3′
-
Has-miR-126 mimics: 5′-UCGUACCGUGAGUAAUAAUGCG-3′ (sense)
-
5′-CAUUAUUACUCACGGUACGAUU-3′ (anti-sense)
-
Mimics negative control: 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense)
-
5′-ACGUGACACGUUCGGAGAATT-3′ (anti-sense)
According to the manufacturer’s protocol, HUVECs were transfected with the miR-126 inhibitor or miR-126 mimics in six-well plates (6 × 105 cells/well) at a final concentration of 100 nM using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Instantly, the cells were incubated at 37 °C in a 5% CO2 atmosphere for 36 h.
In vitro experiments using a HUVEC damage model
Referring to the literature [18], HUVECs were exposed to CoCl2 (250 μM) for 12 h to mimic hypoxic/ischemic conditions and induce a hypoxia damage model. MiR-126 and mRNA level of its target gene PIK3R2 were detected by qRT-PCR. Protein levels of PIK3R2, pS473Akt, pT308Akt, Akt, and PTEN were correspondingly measured by western blotting. Cell apoptosis of HUVECs with different treatments was analyzed using a flow cytometer (Accuri™ C6, BD Biosciences, San Diego, CA, USA) after being stained with PI and FITC-Anexin V.
Statistical analysis
All data were processed with Data Processing System (version 5.5) software and then expressed as average ± SD (standard deviation). The differences between groups were analyzed using one-way ANOVA followed by Fisher’s post hoc test. The statistical difference was considered to be significant if P < 0. 05 or very significant if P < 0. 01.
Results
The repressed miR-126 expression in femoral veins was negatively correlated with DVT
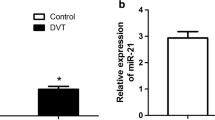
QRT-PCR analysis showed that miR-126 level in femoral veins was descending after quantitative hit, and the differences were significant in all phases (2.5, 5, 15, 25, 35, and 45 h) compared with the normal group. The decreases of miR-126 were very significant between 0 h (normal group) and 2.5 h, and between 15 and 25 h (Fig. 1a), based on which we chose the time points 2.5 and 25 h for the following study.
MiR-126 expression was negatively correlated with DVT. a Relative level of miR-126 in VECs after DVT. b Relative expression of miR-126 in femoral veins after in vivo transfection with miR-126 antagomir or miR-126 agomir. c Representative HE staining of femoral vein of rats in different groups at 25 h after quantitative hit. **P < 0.01, indicated a very significant difference between two groups
Compared with the normal rats, the rats in the model (negative control) groups showed obvious thrombosis in femoral veins at 25 h after quantitative hit (Fig. 1c). But if the rats were pretreated with antagomir-126 (RiboBio, Guangzhou, China), namely the miR-126 was pre-inhibited in vivo (Fig. 1b), more blood clots were observed compared with the negative control group (Fig. 1c). While in the miR-126 overexpression groups where the rats were injected with agomir-126 and miR-126 expression was significantly (P < 0.01, compared with negative controls) upregulated (Fig. 1b), the thrombosis became less serious than that in the negative control group in the same phase (Fig. 1c).
MiR-126 was negatively correlated with VEC apoptosis
Then the apoptosis of vascular endothelial cells in femoral veins was detected using TUNEL assay. In the normal group, no apoptosis of vascular wall cells of femoral vein was observed (Fig. 2a). The results showed that fluorescence intensity (meaning the degree of cell apoptosis) gradually increased over time after the rats were hit, and the intensity was greater at 25 h (Fig. S1, Fig. 2b). In the miR-126 inhibition groups, VEC apoptosis was obviously greater than that in the negative control groups (model 2.5 h and model 25 h) in the same phase (Fig. 2c). While miR-126 overexpression was able to attenuate the apoptosis of vascular endothelial cells, the difference became more notable at 25 h after thrombosis (Fig. 2d).
Apoptosis of VECs in femoral vein from different groups (a normal, b negative control, c miR-126 inhibition, d miR-126 overexpression) detected by TUNEL at 25 h after quantitative hit. The fluorescence intensity was quantitatively analyzed with ImageJ software (e). *P < 0.05, **P < 0.01, compared with negative control; ## P < 0.01, miR-126 inhibition vs. miR-126 overexpression
Differentially expressed MMP-1, -13, and TIMP-1 were reversed by miR-126 inhibition and enhanced by miR-126 overexpression
As shown in Fig. 3, two important MMPs, MMP-1 and MMP-13, showed significantly (P < 0.05) higher protein and mRNA levels during thrombosis after hit, though not all in time-dependent manners. While the protein and mRNA levels of TIMP-1 were significantly (P < 0.05) decreased. When miR-126 was overexpressed, the mRNA and protein levels of the MMPs were significantly (P < 0.05) reduced (compared with negative controls, namely model 2.5 h and model 25 h groups, respectively). If it was inhibited, MMP-1 and MMP-13 expression could significantly exceed the levels in negative controls at a certain time. Compared with miR-126 inhibition groups, the mRNA and protein levels of MMP-1 and MMP-13 in the corresponding miR-126 overexpression groups were remarkably (P < 0.01) lower, but the levels of TIMP-1 were significantly (P < 0.05) higher.
MRNA and protein levels of MMP-1, MMP-13, and TIMP-1 in vascular wall of femoral vein. a MRNA levels of MMP-1, MMP-13, and TIMP-1. b Representative images of western blotting. c Semi-quantitative results of western blot analysis. *P < 0.05, **P < 0.01, compared with normal group; # P < 0.05, ## P < 0.01, compared with model 2.5 h group; & P < 0.05, && P < 0.01, compared with model 25 h group; a P < 0.05, b P < 0.01, miR-126 overexpression vs. miR-126 inhibition
All the above results indicated that miR-126 was negatively correlated with the MMPs and positively correlated with TIMP-1, and the reduced miR-126 was likely to promote cell apoptosis of vascular endothelia and to accelerate thrombosis.
PI3K/Akt signaling transduction was suppressed in DVT-induced cell apoptosis
Twenty-five hours after hit, Akt phosphorylation at S473 was significantly (P < 0.05) inhibited and the inhibition was significantly (P < 0.05) enhanced by miR-126 inhibition, but reversed by miR-126 overexpression (Fig. 4a, b), where the differences were significant (P < 0.01) compared with the negative control groups at the same time point. The phosphorylation of Akt at T308 after quantitative hit appeared to increase, but differences were not found significant (P > 0.05). However, significant differences (P < 0.05) were still found between the negative control groups and the miR-126 inhibition groups or the miR-126 overexpression groups at the same time phase.
Protein levels of pS473Akt, pT308Akt, Akt, Bcl-2, Bad, procaspase-9, and cleaved caspase-9. a Representative western blot images of the proteins. b Semi-quantitative results of protein levels of pS473Akt and pT308Akt. c Semi-quantitative results of protein levels of Bcl-2 and Bad. *P < 0.05 indicated a significant difference, **P < 0.01 indicated a very significant difference
As shown in Fig. 4 (a, c), Bcl-2 level was reducing gradually when thrombosis was happening, and it was negatively correlated with thrombosis and miR-126 expression. But the levels of Bad and cleaved caspase-9, which were the downstream molecules of PI3K/Akt signaling pathway, were both positively correlated with thrombosis, and miR-126 overexpression could significantly reduce them. These results suggested that PI3K/Akt signaling was suppressed in the DVT-induced cell apoptosis, and the suppression was at least partly relieved by the overexpression of miR-126.
MiR-126 expression was negatively correlated with VEC apoptosis in vitro
When HUVECs were cultured and exposed to CoCl2, the expression of miR-126 was reduced and the level of its direct target PIK3R2 was increased. The inhibition of miR-126 by miR-126 inhibitor significantly suppressed miR-126 expression and promoted PIK3R2 expression at both mRNA and protein levels (Fig. 5a). Meanwhile, we also found the apoptosis of HUVECs was induced by CoCl2 and the apoptosis was aggravated by the miR-126 inhibitor and alleviated by miR-126 mimics (Fig. 5b).
CoCl2-induced apoptosis of HUVECs was associated with miR-126 downregulation, and PI3K/Akt suppression in vitro was enhanced by miR-126 inhibition and reversed by miR-126 overexpression. a The RNA expression level of miR-126 and PIK3R2 was decreased after HUVECs were treated with CoCl2 for 12 h, and miR-126 inhibitor/mimics could significantly inhibit/promote the expression of miR-126 and PIK3R2. b The inhibition of miR-126 remarkably accelerated HUVEC apoptosis in vitro, and miR-126 overexpression attenuate the apoptosis. c Representative western blot bands of pS473Akt, pT308Akt, PTEN, and Akt. d Quantitative density analysis results of western blotting. *P < 0.05, **P < 0.01, compared with normal HUVECs (treated without CoCl2, miR-126 inhibitor, or miR-126 mimics). # P < 0.05, ## P < 0.01, compared with the HUVECs only treated with CoCl2
PI3K/Akt suppression in HUVECs injured by CoCl2 was attenuated by miR-126 overexpression and enhanced by miR-126 inhibition
HUVECs were exposed to CoCl2, Akt activation was significantly (P < 0.01) inhibited especially at pS473 (Fig. 5c, d), compared with the group treated without CoCl2. But when the cells were treated with both CoCl2 and miR-126 inhibitor, the inhibition of p-Akts was significantly enhanced (P < 0.05, Fig. 5c, d, b vs. d). However, markedly (P < 0.05) increased levels of Akt phosphorylation was observed in the group treated with miR-126 mimics (Fig. 5c, d, b vs. c). As one of the negative-regulatory factors of PI3K signaling, PTEN expression was found negatively correlated with the phosphorylation of Akts. It was somehow suppressed by miR-126 to help HUVECs escaped from apoptosis. These findings showed us that PI3K/Akt signaling was suppressed in the in vitro HUVECs exposed to CoCl2, and the suppression was enhanced by miR-126 inhibition and attenuated by miR-126 overexpression, which was consistent with the results of the above in vivo experiment.
Discussion
MiR-126, an endothelial-specific intronic product of the vascular endothelial EGF-like 7 gene, is able to govern vascular integrity and angiogenesis, and knockdown of miR-126 will result in loss of vascular integrity and defects in endothelial cell proliferation in vivo [9, 12, 19]. What is more, inhibition of miR-126 can also significantly affect other functions of VECs, cause cell apoptosis, and as a result, promote DVT, which were verified in our present study (Figs. 1 and 2). In thrombosis, miR-126 signals the need for endothelial repair through its transfer from apoptotic endothelial cells [20], where the signal is an intercellular one that can enhance the proliferation, differentiation, and migration of endothelial progenitor cells; therefore, miR-126 is a promoting factor for endothelial regeneration [20, 21]. Based on these previous findings, we think the normal expression of miR-126 is of great significance for endothelial cells’ fate and their regeneration. However, we found in the present study that miR-126 expression was significantly suppressed in DVT, which probably not only accelerated the apoptosis of endothelial cells, but also interdicted their regeneration.
PI3K-dependent Akt activation is able to phosphorylate Bad at Ser136/Ser112, then the phosphorylated Bad is depolymerized from Bcl-2 or Bcl-xL and exert anti-apoptosis effect through combining to 14–3–3 proteins, and meanwhile, the dissociative Bcl-2 can also function as an anti-apoptosis protein [22]. PI3K/Akt activation can suppress cell apoptosis through Bax inactivation by phosphorylating at Ser184 [23] and through the inhibition of caspase activation as well [24]. In this study, we found that miR-126 was reduced after DVT (in vivo; Fig. 1a), and Akt activation was significantly suppressed in both DVT rats and CoCl2-damaged HUVECs (Figs. 4 and 5). Further, the downstream molecules of PI3K/Akt also differentially expressed after DVT. To confirm the role of miR-126 in PI3K/Akt signal pathway, we studied not only the expression changes of phosphorylated Akts after miR-126 inhibition or miR-126 overexpression, but also those of Bcl-2, Bad, and caspase-9. And the results suggested that overexpressed miR-126 in VECs during DVT promoted Akt activation and the expression of the anti-apoptosis protein Bcl-2, and suppressed the proapoptotic activities of Bad and cleaved caspase-9. Currently, it is well known that PIK3R2 is a direct target of miR-126 and a suppressor of the PI3K/Akt signaling pathway as well [15, 25]. Based on these knowledge, we preliminarily concluded that the suppressed PI3K/Akt signaling axis in endotheliocytes is mainly caused by miR-126 inhibition, and miR-126 overexpression may serve as an effective method to suppress apoptosis of VECs and DVT through enhancing the activation of anti-apoptotic pathway PI3K/Akt.
According to the reports [26, 27], MMP-1 and MMP-13 are also the downstream molecules, at least are involved in PI3K/Akt signaling. Probably for these reasons, the MMP-1 and MMP-13 levels were increased and the TIMP-1 level was reduced, the apoptosis of VECs in femoral vein was subsequently aggravated, and finally, thrombogenesis was accelerated (Figs. 1 to 3). The results about the effect of MMPs on thrombosis were consistent with the findings reported by Trivedi et al. [28] and Zhang et al. [29].
VECs apoptosis is in the core of DVT pathogenesis, and therefore, it is a very promising therapeutic target. MiR-126 has a variety of functions in the development of thrombosis and atherosclerosis, including mediating both the intracellular and intercellular apoptotic signals in and out of VECs [19, 20, 30]. Here, we validated that the anti-apoptosis pathway PI3K/Akt was regulated by miR-126 in VECs, and mainly in this way, overexpressed miR-126 decreased the apoptosis of VECs. Additionally, PTEN expression was also suppressed by miR-126, so PI3K signaling was enhanced to resist against HUVEC apoptosis (Fig. 5). This research explored the effect of miR-126 and the underlying mechanism in DVT at cell and animal levels, which is of significance for clarifying the role of miR-126 in DVT pathogenesis, and it may provide a new target for the therapy of DVT.
References
Office of the Surgeon General (US), National Heart L, and Blood Insititue (US) (2008) The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. Office of the Surgeon General (US), Rockville (MD)
Fu Y, Wang D (2006) Emergency care and prevention of thromboembolic disease. Chinese Journal for Clinicians 34(8):51–53
Shyy JY-J, Chien S (2002) Role of integrins in endothelial mechanosensing of shear stress. Circ Res 91(9):769–775
Bombeli T, Karsan A, Tait JF, Harlan JM (1997) Apoptotic vascular endothelial cells become procoagulant. Blood 89(7):2429–2442
Mo J, Huang H, He F, Zhang C, Zhao X, Li S (2007) Influence of Apoptosis Signal Pathway in Traumatic Deep Vein Thrombosis. Journal of Kunming Medical University 28(5):5–7
D’haene B, Mestdagh P, Hellemans J, Vandesompele J (2012) miRNA expression profiling: from reference genes to global mean normalization. In: Next-Generation MicroRNA Expression Profiling Technology. Springer, pp 261–272
Zhang E, Wu Y (2013) MicroRNAs: important modulators of oxLDL-mediated signaling in atherosclerosis. J Atheroscler Thromb 20(3):215–227
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN (2008) The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 15(2):261–271
Musiyenko A, Bitko V, Barik S (2008) Ectopic expression of miR-126*, an intronic product of the vascular endothelial EGF-like 7 gene, regulates prostein translation and invasiveness of prostate cancer LNCaP cells. J Mol Med 86(3):313–322
Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ (2008) MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A 105(5):1516–1521
Liu B, Peng X-C, Zheng X-L, Wang J, Qin Y-W (2009) MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer 66(2):169–175
Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15(2):272–284
Mo J, Huang H, Zhang C, Wang B, He F, Yin L, Zhao Z, Tang X, Zhou Z, Zhao X (2007) Influence of JAK-STAT signaling pathway in traumatic deep vein thrombosis. Chinese Journal of Misdiagnostics 7(26):6212–6214
X-q S, Xu Z-m, Xie M-b, D-a P (2014) Resveratrol inhibits hydrogen peroxide-induced apoptosis in endothelial cells via the activation of PI3K/Akt by miR-126. J Atheroscler Thromb 21(2):108–118
Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen C-Z, Kuo CJ (2008) Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development 135(24):3989–3993
Wang C-Y, Tsai A-C, Peng C-Y, Chang Y-L, Lee K-H, Teng C-M, Pan S-L (2012) Dehydrocostuslactone suppresses angiogenesis in vitro and in vivo through inhibition of Akt/GSK-3β and mTOR signaling pathways. PLoS One 7(2):e31195
Tsuzaki M, Guyton G, Garrett W, Archambault J, Herzog W, Almekinders L, Bynum D, Yang X, Banes A (2003) IL‐1β induces COX2, MMP‐1,‐3 and‐13, ADAMTS‐4, IL‐1β and IL‐6 in human tendon cells. J Orthop Res 21(2):256–264
Cho CM, Ha SU, Bae HR, Huh JT (2006) Endothelial cell products as a key player in hypoxia-induced nerve cell injury after stroke. J Korean Neurosurg Soc 40:103–109
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN (2008) The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 15(2):261–271. doi:10.1016/j.devcel.2008.07.002
Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A (2013) MicroRNA-126,-145, and-155 A Therapeutic Triad in Atherosclerosis? Arterioscler, Thromb, Vasc Biol 33(3):449–454
Yan T, Liu Y, Cui K, Hu B, Wang F, Zou L (2013) MicroRNA‐126 regulates EPCs function: Implications for a role of miR‐126 in preeclampsia. J Cell Biochem 114(9):2148–2159
Henshall DC, Araki T, Schindler CK, Lan J-Q, Tiekoter KL, Taki W, Simon RP (2002) Activation of Bcl-2-associated death protein and counter-response of Akt within cell populations during seizure-induced neuronal death. J Neurosci 22(19):8458–8465
Xin M, Deng X (2005) Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem 280(11):10781–10789
Shultz JC, Goehe RW, Wijesinghe DS, Murudkar C, Hawkins AJ, Shay JW, Minna JD, Chalfant CE (2010) Alternative splicing of caspase 9 is modulated by the phosphoinositide 3-kinase/Akt pathway via phosphorylation of SRp30a. Cancer Res 70(22):9185–9196
Meng Q, Wang W, Yu X, Li W, Kong L, Qian A, Li C, Li X (2015) Upregulation of MicroRNA‐126 Contributes to Endothelial Progenitor Cell function in deep vein thrombosis via Its Target PIK3R2. J Cell Biochem 116(8):1613–1623
Forough R, Weylie B, Collins C, Parker JL, Zhu J, Barhoumi R, Watson DK (2005) Transcription factor Ets-1 regulates fibroblast growth factor-1-mediated angiogenesis in vivo: role of Ets-1 in the regulation of the PI3K/AKT/MMP-1 pathway. J Vasc Res 43(4):327–337
Dey JH, Bianchi F, Voshol J, Bonenfant D, Oakeley EJ, Hynes NE (2010) Targeting fibroblast growth factor receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs mammary tumor outgrowth and metastasis. Cancer Res 70(10):4151–4162
Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A (2009) Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell 137(2):332–343
Zhang YB, Li W, Yao LQ, Zhao XL, Wang B, Li HK, Ning Y, Song E, Zhang XX (2010) Expression changes and roles of matrix metalloproteinases in a rat model of traumatic deep vein thrombosis. Chinese journal of traumatology = Zhonghua chuang shang za zhi / Chinese Medical Association 13(3):188–192
Sun X, Zhang M, Sanagawa A, Mori C, Ito S, Iwaki S, Satoh H, Fujii S (2012) Circulating microRNA-126 in patients with coronary artery disease: correlation with LDL cholesterol. Thromb J 10(1):16
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by Animal Ethical Committee of Kunming Medical University, and all procedures performed in this study involving animals were conducted strictly according to the official guidance.
Funding
This study is financially supported by Associated Project of Yunnan Province Science & Technology Department and Kunming Medical University Basic Research for Application (No.: 2013FB148).
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 4590 kb)
Rights and permissions
About this article
Cite this article
Chen, L., Wang, J., Wang, B. et al. MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol 95, 365–374 (2016). https://doi.org/10.1007/s00277-015-2567-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2567-9