Abstract
Monitoring of anti-coagulation with the direct factor Xa inhibitor rivaroxaban is considered unnecessary in a routine clinical setting. However, assessment of its anti-coagulant effect may be desirable in certain clinical situations. We assessed prothrombin time (PT) reagents and commercially available anti-Xa assays (Biophen) calibrated for rivaroxaban and heparin in comparison to liquid chromatography–mass spectrometry (LC-MS/MS) measurements of rivaroxaban concentration in samples from patients on treatment with rivaroxaban for stroke prevention in atrial fibrillation. Citrate plasma samples were obtained from 30 randomly selected patients on uninterrupted treatment with rivaroxaban for a minimum of 1 month. The anti-Xa assays, direct Xa inhibitor (DiXa-I®), and Heparin LRT® were conducted for both wide and low calibrations for rivaroxaban. Measurements were compared to LC-MS/MS using correlation, linear regression, intra-class correlation, and Bland–Altman analysis. In 30 patients (9 female) of median age 71.5 years and BMI 26.5 kg/m2, rivaroxaban concentrations between 2.4 and 625 ng/ml (median 82 ng/ml) were measured by LC-MS/MS. PT reagents were poorly correlated with rivaroxaban concentrations (r 2 = 0.52 and 0.09). Anti-Xa assays DiXa-I (r 2 = 0.95) and Heparin LRT (r 2 = 0.97) were correlated with rivaroxaban in all concentrations, but especially in low concentrations with low calibrations (r 2 = 0.97 and 0.98, respectively). The highest agreement occurred between Heparin LRT and low rivaroxaban concentrations with a mean difference of −5.3 ng/ml (limits of agreement, 12.9 to 2.4 ng/ml). Anti-Xa assays can indirectly determine the concentration of rivaroxaban for a wide range of concentrations in real-world patients. An interpretation of anti-Xa and PT measurements in treatment with rivaroxaban requires knowledge of the local reagents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rivaroxaban is a direct inhibitor of coagulation factor Xa, approved by the European Medicines Agency and the US Food and Drug Administration for the treatment and secondary prevention of venous thromboembolism (VTE), stroke prevention in non-valvular atrial fibrillation, and primary VTE prevention after major orthopedic surgery. Contrary to anti-coagulation with vitamin K antagonists, treatment with non–vitamin K antagonist oral anti-coagulants (NOACs) such as rivaroxaban does not require regular monitoring or dose adjustment [1] and reaches a typical concentration of 41–60 ng/ml in trough and 219–305 ng/ml in peak [2]. However, a precise measurement to determine rivaroxaban therapy may be advantageous in certain clinical situations, including the accumulation of rivaroxaban in kidney failure, accidental or intentional overdose, unclear duration of treatment interruption prior to elective surgery, before thrombolysis in ischemic stroke, or in acute critical bleeding with unknown influence of rivaroxaban. [3, 4]. In these situations, decisions could be aided by the results of anti-Xa assays with appropriate calibrations.
The most precise method of measuring plasma concentrations of rivaroxaban is high-performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) [5, 6], a method regrettably not readily available in routine practice. In previous studies, a correlation between plasma concentrations of rivaroxaban and chromogenic anti-Xa assays has been described [6]. Anti-Xa activity is measured by adding a known amount of excess Xa to a sample of unknown rivaroxaban concentration. The residual, uninhibited Xa then cleaves a chromogenic substrate. The absorbance can be measured and is inversely proportional to the unknown concentration of rivaroxaban in the sample. The correlation between the chromogenic anti-Xa method and rivaroxaban concentration was reported to be linear over an on-treatment range of rivaroxaban from approximately 20 to 660 ng [7–9], and low concentrations could be more accurately measured if calibrations were chosen for low concentration ranges [10].
The aims of this investigation were to compare anti-Xa assays and calibrations for rivaroxaban and to provide an accurate assessment of rivaroxaban concentrations from real-world patients with a wide range of concentrations by LC-MS/MS, which will allow interpretation of anti-Xa assays and prothrombin time results for given rivaroxaban concentrations.
Materials and methods
Patients
Clinical plasma samples were obtained from 30 randomly selected patients with a diagnosis of non-valvular atrial fibrillation on treatment with rivaroxaban (Xarelto®, Bayer Pharma AG, Wuppertal, Germany), who visited the outpatient clinic of the Clinical Division of Hematology and Hemostaseology for routine consultation and assessment of their anti-coagulant treatment. The study was approved by the local ethics committee (EC 1711/2014) and is in accordance with the principles set forth by the declaration of Helsinki. Blood was obtained from venous puncture without stasis of the cubital vein using a 21-gauge butterfly needle into a vacuum tube containing trisodium citrate 3.8 % (Vacuette® Greiner Bio-One, Kremsmünster, Austria; 9:1 v/v) as part of routine analysis. Within 1 h after sampling, citrate blood vials were centrifuged at 2500 × g for 15 min at 18 °C and the supernatant platelet-poor plasma (PPP) immediately stored in aliquots at −80 °C until assessment. For the present study, surplus plasma samples were used. Patients’ medical histories were recorded as part of the routine stroke risk and bleeding risk assessments. Serum creatinine levels were obtained from routine blood analysis and calculated to estimate glomerular filtration rate according to Cockcroft–Gault and IDMS-traceable MDRD equations. Time of last rivaroxaban administration was precisely assessed and recorded.
LC-MS/MS
Rivaroxaban quantification by LC-MS/MS was facilitated by utilizing a previously established two-dimensional chromatography setup (Agilent Technologies, Waldbronn, Germany) hyphenated to an API4000Qtrap instrument (ABSciex, Framingham, MA, USA) operated in the electrospray ionization (ESI) mode [11]. Based on this instrumentation, a research use only (RUO) assay was developed and validated. A protein precipitation protocol was used to prepare calibrators, quality control, and patient samples. Aliquots of 150 μl were combined with 400 μl precipitation solution [0.1 M ZnSO4/MeOH = 1:2 (v/v)] spiked with the stable isotope labeled internal standard rivaroxaban-13C6 (Alsachim, Illkirch, France). After homogenization and centrifugation, a 50-μl aliquot of the sample supernatant was transferred to the LC-MS/MS instrument. Residual matrix constituents were separated from the analyte by combining online solid-phase extraction (SPE) purification as first chromatographic dimension with solvent gradient elution over reversed phase C-18 material as second purification step. Analyte elution from the LC system (2.1-min retention time) was detected in the mass spectrometer by monitoring ion pair transitions 436.1 to 145.0 m/z (rivaroxaban quantifier), 436.1 to 231.1 m/z (rivaroxaban qualifier), and 442.1 to 145.0 m/z (rivaroxaban internal standard) in the selected reaction monitoring (SRM) mode. Rivaroxaban quantification was based on external calibration with rivaroxaban (Alsachim) utilizing the above mentioned internal standard. A calibrator set (nine levels, one blank) covering the measurement range of 1.0 (lower limit of quantification [LOQ]) to 600.0 ng/ml (upper LOQ) was used; a linear calibration function with a 1/x weighting factor was employed. Samples with concentrations exceeding the upper LOQ were diluted with blank materials; samples with concentrations below the lower LOQ were not reported. The inter-batch (n = 10) coefficient of variation of the assay was found to be better than 4.7 % for all quality control materials (4, 40, and 400 ng/ml); the accuracy of these measurements was better than ±4.9 %. In patient samples, inter-batch coefficients of variation better than 7.6 % were found.
Coagulation assays
The prothrombin time (PT) was measured using two reagents. The Thromborel S® (Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany) derived from human placenta and the Normotest® (Technoclone GmbH, Vienna, Austria) of combined thromboplastin of rabbit brain and bovine plasma. The PT was measured in the routine coagulation laboratory of the Department of Medical and Chemical Laboratory Diagnostics of the Medical University of Vienna using an accredited process.
Two different anti-Xa assays (provided by CoaChrom Diagnostica GmbH, Maria Enzersdorf, Austria) were compared. BIOPHEN DiXa-I® and BIOPHEN Heparin LRT® (Hyphen–Biomed, Neuville–sur–Oise, France) were both calibrated for a wide and a low range of rivaroxaban concentrations according to the manufacturer’s specifications. Three-point calibration curves were constructed using the commercially available calibrators (Rivaroxaban Plasma Calibrator, CoaChrom Diagnostica) with concentrations 0, 276, and 497 ng/ml for the wide concentration range and 0, 55, and 102 ng/ml for the low concentration range. Calibrations resulted in linear calibration lines over the respective calibration ranges. For each calibration, two control measurements were performed in duplicate using Rivaroxaban Control Plasma (CoaChrom Diagnostica).
The BIOPHEN Heparin LRT® assay was also calibrated with commercially available low molecular weight heparin (LMWH) calibrators in a separate run using a five-point calibration curve of the concentrations 0.00, 0.38, 0.77, 1.18, and 1.51 U/ml. Control measurements were performed with LMWH concentrations 0.24 and 0.86 U/ml and unfractionated heparin (UFH) concentration 0.60 U/ml.
For the measurements, the blinded PPP samples were thawed in a water bath at 38 °C for 10 min and diluted with Tris-NaCl-EDTA buffer in the proportions 1:50 for DiXa-I, 1:20 for DiXa-I low calibration, 1:10 for Heparin LRT rivaroxaban calibration, 1:2 for Heparin LRT low rivaroxaban calibration, and 1:2 for Heparin LRT with heparin calibration. Absorbance was measured at 405 nm on a STA-R Coagulation Analyser (Diagnostica Stago, Asnières sur Seine, France) according to manufacturer instructions.
Statistical methods
Statistical analyses were performed using SPSS (Windows Version 22.0, Armonk, NY: IBM Corp.). The patient cohort was described by their median, range, and/or the 25th to 75th percentile for continuous variables and by absolute and relative frequencies for categorical variables. Measurements of LC-MS/MS rivaroxaban concentration and anti-Xa assays were compared by Spearman correlation, linear regression, and Bland–Altman agreement analysis [12]. Bland–Altman analysis was not feasible for the agreement between PT and rivaroxaban concentrations as well as between Heparin LRT calibrated for heparin and rivaroxaban concentrations due to a difference in scale. The limits of agreement of the Bland–Altman analyses were calculated by the mean difference between the two measurements ± 1.96 × standard deviation of the difference between the two measurements for 5th and 95th percent limit of agreement, respectively. Potential proportional bias in the regression analysis was analyzed by visual analysis of the Bland–Altman plot and a paired-samples t test with p < 0.05. Inter-rater reliability (IRR) between LC-MS/MS and anti-Xa assays was tested with a two-way mixed, absolute agreement, intra-class correlation coefficient (ICC). Prothrombin time reagents were not analyzed by inter-rater reliability because of differences in scale, but instead, a linear regression model was applied. Limit of detection (LOD) of anti-Xa assays for both calibrations was calculated according to LOD = 3.3 × SD / k and limit of quantification (LOQ) according to LOQ = 10 × SD / k, where SD is the residual standard deviation and k is the slope of the curve constructed from calibrators.
Results
Patient cohort
Plasma samples from 30 patients on treatment with rivaroxaban were included in the investigation. Patients were in median 71.5 years of age and had a median BMI of 26.5 kg/m2, and 9 patients (30 %) were female. At the time of sampling, all patients were on treatment with rivaroxaban after a median time on treatment of 4.8 months and minimum time of 1 month on rivaroxaban. Twenty-one patients (70 %) received 20 mg rivaroxaban once daily (OD), eight patients (26.6 %) received 15 mg rivaroxaban OD, and one patient (3.3 %) received 10 mg rivaroxaban OD temporarily given by the treating physician due to a transient high risk of bleeding in an off-label dosage. Time of last drug administration was in median 2.9 h before sampling. Concentrations of rivaroxaban measured by LC-MS/MS reached 82 ng/ml in median (25th to 75th percentile 22.4–204.0 ng/ml) and a concentration range from 2.4 to 625 ng/ml. Detailed patient characteristics are provided in Table 1. The concentrations of rivaroxaban in 30 patient samples and corresponding time since drug administration are provided in Fig. 1.
A subgroup of 17 patients with rivaroxaban concentrations below 102 ng/ml was selected to compare coagulation assays in a low concentration range. The median rivaroxaban concentration in this subgroup was 29.6 ng/ml (25th to 75th percentile 8.7–46.9 ng/ml) and the median time since last drug administration was 15.5 h (25th to 75th percentile 1.9–24.8 h, Table 1). In patients excluded from this subgroup, all anti-Xa assays reliably measured a concentration above the upper calibration point for the low concentration range (data not shown).
Prothrombin time
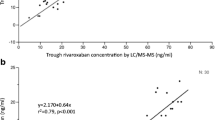
A median PT of 15.5 and 22.6 s was measured with Thromborel S and Normotest reagents, respectively. The correlation between PT and rivaroxaban concentration was moderate using both Thromborel S and Normotest reagents in patient samples with a rivaroxaban concentration ranging between 2.4 and 625 ng/ml (Table 2). The linear regression coefficient of determination r 2, however, revealed that the PT using either reagent is a poor measure of rivaroxaban concentration (Table 2). Patient samples that were within the local reference range for a normal PTwith the Thromborel S reagent (11.0–14.0 s) had corresponding rivaroxaban concentrations from 2.4 to 66 ng/ml (n = 8). Patient samples within the normal reference range for the Normotest reagent (16.7–22.8 s) had corresponding rivaroxaban concentrations from 2.4 to 125 ng/ml (n = 12).
Chromogenic anti-Xa assay Biophen DiXa-I
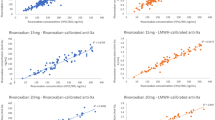
In a comparison of all patient samples, the Spearman correlation between Biophen DiXa-l and LC-MS/MS was strong (rho 0.98, p < 0.001; linear regression r 2 = 0.95). In the Bland–Altman analysis, a mean difference of −11.3 ng/ml (5th and 95th percent limit of agreement −79.4 to 56.8 ng/ml) was found between DiXa-I and LC-MS/MS. No proportional bias (p > 0.05) was found in the analysis of difference between the measures in the Bland–Altman plot provided in Fig. 2. An excellent intra-class correlation coefficient (ICC) of 0.98 (95 % confidence interval (CI) 0.97–0.99) was found in the reliability analysis. In patient samples with a low drug concentration, DiXa-I measurements correlated very well with LC-MS/MS measurements of rivaroxaban and even improved slightly with calibrations for lower concentration range (Table 2). The 5th and 95th percent limits of agreement decreased to −21.0 and 4.1 ng/ml when recalibrated for low concentration ranges. This indicates lower imprecision of the anti-Xa assay when calibrated for low concentration ranges (Fig. 3). A potential source of bias for the low calibration range was identified in the Bland–Altman analysis as the low calibrator slightly overestimated low rivaroxaban concentrations (p < 0.001). The LOD and LOQ for Biophen DiXa-I improved from 35.4 and 107.3 ng/ml, respectively, to 9.1 and 27.8 ng/ml with low calibrations.
Concentration measurement of Biophen DiXa-I® plotted against LC-MS/MS measurement for the wide calibration of rivaroxaban concentrations and Bland–Altman analysis of agreement (n = 30). Anti-Xa and LC-MS/MS measurements are both given in ng/ml rivaroxaban. The limits of agreement of the Bland–Altman analysis were calculated by the mean difference between the two measurements ± 1.96 × standard deviation of the difference between the two measurements
Chromogenic anti-Xa assay Biophen Heparin LRT
The Spearman correlation coefficient between rivaroxaban-calibrated Biophen Heparin LRT and LC-MS/MS in all samples from patients on rivaroxaban treatment was 0.99 (p < 0.001; linear regression r 2 = 0.97). In the Bland–Altman analysis, a mean difference of 0.4 ng/ml (5th and 95th percent limits of agreement −54.1 to 54.9 ng/ml) was found between Heparin LRT and LC-MS/MS measurements. In the Bland–Altman plot, provided in Fig. 4, the scatter of differences increases with increasing mean of LC-MS/MS and Heparin LRT. This bias, however, disappears in the natural log transformation of the Bland–Altman plot (detailed data not shown, but can be provided upon request). The reliability analysis shows excellent agreement with an ICC of 0.99 (95 % CI 0.98–1.0).
In patient samples with a low drug concentration, agreement between Heparin LRT and LC-MS/MS for both low and wide concentration ranges was very good, but the 5th and 95th percent limits of agreement decreased to −12.9 and 2.4 ng/ml, respectively, when the low calibration was used (Figs. 4 and 5). Heparin LRT wide and low calibrations were also slightly biased toward overestimating rivaroxaban concentrations, especially in very low concentrations with a p < 0.05 in the paired-samples t test of the Bland–Altman analysis (Figs. 4 and 5). The LOD and LOQ of Heparin LRT improved from 49.1 and 148.7 ng/ml, respectively, to 2.1 and 6.3 ng/ml.
In separate runs of the Biophen Heparin LRT with a calibration for LMWH, measurements correlated well with the lower concentrations of rivaroxaban (Spearman correlation coefficient 0.98, p < 0.001, linear regression r 2 = 0.98, p < 0.001, Table 2) but reached the upper limit of quantification of 1.5 U/ml at a rivaroxaban concentration of approximately 101 ng/ml.
Discussion
A measurement of rivaroxaban concentration or activity is generally considered unnecessary in a routine setting of continuous anti-coagulation therapy for non-valvular atrial fibrillation or VTE treatment and prevention [1, 4, 13]. The advantageous pharmacokinetic and pharmacodynamic profile of rivaroxaban and other NOACs provides clinicians with a good option for treating patients with need for anti-coagulation without routine treatment monitoring. Despite the advantages of rivaroxaban, a need for measurement has been expressed in certain clinical and especially critical situations.
Our LC-MS/MS concentration measurements confirmed previous findings of a large inter-individual variability of rivaroxaban concentrations [8], especially during the first 4 h after drug administration. The nature of this variability may originate from the resorption properties of rivaroxaban. The LC-MS/MS method provides an exact concentration measurement of rivaroxaban [5]. For instance, in our study of randomly selected real-world patients under rivaroxaban treatment, we reached a LOQ of 1 ng/ml and an upper limit of quantification of 600 ng/ml with a coefficient of variation of 4–7 %.Unfortunately, LC-MS/MS is not available in most coagulation laboratories, and most importantly, this method is not available on short notice, which hampers its routine clinical use.
Chronometric coagulation measures, which were developed for VKA and heparins, such as the aPTT and the PT, are not or not reliably affected by rivaroxaban to provide a convincing diagnostic tool [14]. Although, overall, the PT correlated with the rivaroxaban concentration in our study, it was not sensitive to rivaroxaban in clinically relevant concentrations for neither of the reagents used. Our findings on the PT are in agreement with previous studies [6, 14–16], and we were able to show that a normal PT, measured with either the Normotest or the Thromborel S reagent, does not exclude clinically relevant concentrations of rivaroxaban and is dependent on the reagents used in the local coagulation laboratory [8]. In analogy to the findings by Hillarp et al., we found the Quick-type Thromborel S assay slightly superior to the Normotest assay [16], possibly due to a different composition of thromboplastin.
Chromogenic anti-Xa assays were proposed to give a very precise measurement of the rivaroxaban concentration in plasma when calibrated for rivaroxaban [2, 6, 8, 10, 9]. Using LMWH or UFH calibrated anti-Xa assays may provide a crude estimate of the rivaroxaban concentration present given knowledge and experience with the local reagents and calibrations. The results of our coagulation analyses provide an assessment of real-world patient samples on treatment with rivaroxaban rather than of rivaroxaban-spiked plasma. We were able to show a clear correlation between LC-MS/MS and the anti-Xa assays Biophen DiXa-I and Biophen Heparin LRT calibrated for rivaroxaban.
Further, we could demonstrate that in patients with rivaroxaban concentrations below 102 ng/ml, use of the low calibrators for the anti-Xa assays slightly improved the correlation, the linear regression, and the inter-rater reliability with the LC-MS/MS measurement (Table 2). In the Bland–Altman analysis, the anti-Xa assays for both low and wide calibrations, but most prominently in low concentrations, slightly overestimated drug concentration resulting in an overall negative mean difference. A proportional bias was discovered in the Heparin LRT and the low calibration of the DiXa-I assays, where the true concentration of rivaroxaban was systematically overestimated. Despite log transformation of the measurements, especially in the lowest concentrations of rivaroxaban, the anti-Xa assays and the LC-MS/MS method deviated the most. We assume that in low concentrations, the anti-Xa activity is more severely disturbed by endogenous factors than in high rivaroxaban concentrations. Also, with increasing specificity of the assays, inter-individual variability between real-world patient samples becomes more apparent. The LOD and LOQ of anti-Xa assays have been assessed in previous studies to lie at approximately 10 and 30 ng/ml, respectively [2, 9]. The slight deviation of the LOD in our results from previous findings may be attributed to the differences in statistical methods used.
Based on our results, we have to consider that in a critical situation where a very high concentration of rivaroxaban has to be excluded, such as accidental or intentional overdose or accumulation in kidney failure, a calibration for a wide range of rivaroxaban concentrations would seem appropriate. A PT result within a normal range can possibly rule out a high concentration of rivaroxaban present but is highly dependent on local reagents and thus requires some experience with local circumstances [17]. In clinical situations, where a low but relevant concentration has to be confirmed, such as planned surgery with assumed residual rivaroxaban despite adequate temporary interruption, before thrombolysis in ischemic stroke patients, or an acute critical situation with unknown last administration of rivaroxaban, a calibration for low ranges of rivaroxaban would seem diagnostically most conclusive. A measurement of rivaroxaban anti-coagulation during the first 3–4 h after drug administration with any assay should be interpreted with the knowledge that rivaroxaban concentration, like other NOACs [18], may still be increasing (depicted in Fig. 1). When calibrated for LMWH, the anti-Xa assay may indicate the presence of clinically relevant concentrations of rivaroxaban, only if the presence of LMWH or UFH can be excluded with certainty.
Our results contribute to the evidence on how to measure and interpret the treatment with rivaroxaban in real-world patients. We are, however, aware of some limitations. The administration of rivaroxaban was not controlled in our study. Samples were collected from clinical surplus citrate plasma and the information on the time of drug administration was reported by the patients. We believe, however, that our cohort relates to the clinical reality of anti-coagulation treatment practice. Given the relatively small number of patients, the coagulation measurements may be biased or lack discriminatory power. We attempted to reduce bias by conducting LC-MS/MS and anti-Xa measurements without knowledge of the reciprocal results, patient characteristics, or time of drug administration. We did not perform measurements in samples with other NOACs such as apixaban or edoxaban and cannot assess how our findings extend to other direct inhibitors of FXa. Nonetheless, we believe in our investigation of coagulation assays that the use of real-world patient samples was advantageous to the use of drug-spiked normal plasma because we were able to capture more characteristics of the heterogeneous population of anti-coagulated patients.
Conclusion
With more specific anti-Xa assays, such as the Biophen DiXa-I and specific calibrators for low or wide concentration ranges, the agreement with the LC-MS/MS measured rivaroxaban concentration can be very precise. PT is an insufficient measure to exclude relevant concentrations of rivaroxaban. In order to have confidence in the interpretation of the coagulation tests, however, a communication of the clinical situation and the time since last drug administration with the local coagulation laboratory is important.
References
Kitchen S, Gray E, Mackie I, Baglin T, Makris M (2014) Measurement of non-Coumarin anticoagulants and their effects on tests of Haemostasis: guidance from the British Committee for Standards in Haematology. Br J Haematol 166:830–841
Cuker A, Siegal DM, Crowther MA, Garcia DA (2014) Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 64:1128–1139
Ten Cate H (2013) New oral anticoagulants: discussion on monitoring and adherence should start now. Thromb J 11:8
Samama MM, Guinet C, Le Flem L (2012) Do new oral anticoagulants require laboratory monitoring? The clinician point of view. Thromb Res 130(Suppl):S88–S89
Rohde G (2008) Determination of rivaroxaban–a novel, oral, direct Factor Xa inhibitor–in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 872:43–50
Douxfils J, Tamigniau A, Chatelain B, Chatelain C, Wallemacq P, Dogné J-M, Mullier F (2013) Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost 110:723–731
Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, Le Flem L, Rohde G, Martinoli JL (2012) Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 107:379–387
Francart SJ, Hawes EM, Deal AM, Adcock DM, Gosselin R, Jeanneret C, Friedman KD, Moll S (2014) Performance of coagulation tests in patients on therapeutic doses of rivaroxaban. A cross-sectional pharmacodynamic study based on peak and trough plasma levels. Thromb Haemost 111:1133–1140
Schmitz EM, Boonen K, van den Heuvel DJ, van Dongen JL, Schellings MW, Emmen JM, van der Graaf F, Brunsveld L, van de Kerkhof D (2014) Determination of dabigatran, rivaroxaban and apixaban by UPLC-MS/MS and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J Thromb Haemost 12:1636–1646
Mani H, Rohde G, Stratmann G, Hesse C, Herth N, Schwers S, Perzborn E, Lindhoff-Last E (2012) Accurate determination of rivaroxaban levels requires different calibrator sets but not addition of antithrombin. Thromb Haemost 108:191–198
Seger C, Tentschert K, Stöggl W, Griesmacher A, Ramsay S (2009) A rapid HPLC-MS/MS method for the simultaneous quantification of cyclosporine A, tacrolimus, sirolimus and everolimus in human blood samples. Nat Protoc 4:526–534
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P (2013) European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 15:625–651
Dale BJ, Ginsberg JS, Johnston M, Hirsh J, Weitz JI, Eikelboom JW (2014) Comparison of the effects of apixaban and rivaroxaban on prothrombin and activated partial thromboplastin times using various reagents. J Thromb Haemost 12:1810–1815
Mani H, Hesse C, Stratmann G, Lindhoff-Last E (2011) Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost 106:156–164
Hillarp A, Baghaei F, Fagerberg Blixter I, Gustafsson KM, Stigendal L, Sten-Linder M, Strandberg K, Lindahl T (2011) Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost 9:133–139
Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE (2010) Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit 32:673–679
Halbmayer W-M, Weigel G, Quehenberger P, Tomasits J, Haushofer AC, Aspoeck G, Loacker L, Schnapka-Koepf M, Goebel G, Griesmacher A (2012) Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med 50:1601–1605
Acknowledgments
We thank the clinical collaborators Johannes Thaler, Johanna Gebhart (Department of Hematology and Hemostaseology), Giora Meron (Department of Emergency Medicine/Ophthalmology), Martin Frossard (Department of Emergency Medicine/Trauma Surgery), and Christoph Kratochwill (Department of Internal Medicine II, all Medical University of Vienna) for contributing patient samples. The anti-Xa reagents, calibrators, and controls were kindly provided by CoaChrom Diagnostica GmbH, Maria Enzersdorf, Austria.
Funding
This investigation was supported by an unrestricted grant from Daiichi-Sankyo Austria.
Conflict of interest
The authors declare that they have no conflicts of interest.
Authors contribution
OK acquired data, analyzed and interpreted data, drafted the manuscript, and approved the final version for publication.
SB acquired data, analyzed and interpreted data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
PQ analyzed and interpreted data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
CS designed and validated the LC-MS/MS platform, acquired data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
GW acquired data, critically revised the manuscript for important intellectual content and approved the final version for publication.
AG acquired data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
IP designed and conceived the study interpreted data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
CA designed and conceived the study, analyzed and interpreted data, critically revised the manuscript for important intellectual content and, approved the final version for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Königsbrügge, O., Quehenberger, P., Belik, S. et al. Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann Hematol 94, 1463–1471 (2015). https://doi.org/10.1007/s00277-015-2407-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-015-2407-y