Abstract
Determination of donor chimerism profiles in blood or bone marrow from patients with allogeneic stem cell transplantation (SCT) is useful for monitoring engraftment or predicting relapse, when specific molecular markers are lacking. CD34+ donor cell chimerism (DCC) analysis in peripheral blood samples from CD34+ acute myeloid leukemia (AML) and myleodysplastic syndrome (MDS) patients proved to be a highly sensitive diagnostic tool that is useful to detect imminent relapse significantly earlier compared to total white blood cell donor cell chimerism monitoring. However, flow-cytometric enrichment of CD34+ cells requires high efforts to human resources and equipment. We present a novel semi-automated CD34+ DCC analysis procedure—employing a magnetic cell-enrichment device, DNA extraction, and short tandem repeat profiling—without the need for flow-cytometric cell sorting. Monitoring 85 patients with AML and MDS over a period of 4 years 24 relapses were detected. Semi-automated peripheral blood CD34+ DCC was diminished below 80 % in all cases of systemic relapse. Significant decrease of the CD34+ DCC value was detected 29–42 days before overt cytological relapse. Our method provides a rapid and sensitive tool for monitoring AML and MDS patients after allogeneic SCT with regard to engraftment and early detection of relapse. Here, we propose a novel semi-automated procedure for CD34+ DCC analysis after allogeneic SCT that is simple, reliable, and therefore applicable in all hematologic laboratories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For patients with acute myeloid leukemia (AML), the allogeneic stem cell transplantation (SCT) currently represents the treatment strategy with the best prognosis in intermediate and high-risk AML [1, 2], but relapse is still a major cause of death [3]. Leukemia-specific genetic aberrations or a leukemia-specific aberrant immunophenotype are currently in use for monitoring minimal residual disease (MRD) and pending relapse [4]. Quantitative polymerase chain reaction (PCR) and multiparameter flow cytometry are highly sensitive methods for MRD detection. If those methods are not applicable, CD34+ donor cell chimerism (DCC) analysis proved to be a reliable and sensitive method for the detection of imminent relapse after allogeneic SCT [5, 6]. Decline of the donor signal in CD34+ DCC predicts inferior survival because treatment of post-transplantation relapses is difficult [7]. Nevertheless, treatment strategies such as cessation of immunosuppression, low-dose Ara-C, donor lymphocyte infusion (DLI) with application of GM-CSF and/or secondary transplantation can still rescue some of these patients with recurrent AML after SCT [8]. Platzbecker et al. recently suggested azacytidine treatment for patients with a decline of the CD34+ donor cell chimerism below 80 % [9]. Close follow-up care provides a window for therapeutic intervention with more effective treatment, than the overt relapse [10] and disease control by low-dose Ara-C prior donor cell transfusion is predictive for better survival in patients [8]. We had previously reported that sequential chimerism analysis in CD34+ cells from peripheral blood is sensitive, allowing the early detection of relapse and monitoring of therapeutic intervention [11]. CD34+ DCC is applicable in about 80 % of AML patients, when the blast population expresses the CD34 surface antigen [7, 12]. However, the method of combined density centrifugation, magnetic cell-enrichment, and subsequent flow cytometry-based cell sorting of peripheral blood CD34+ cells prior to short tandem repeat (STR)-profiling as reported is cumbersome, expensive, and requires a peripheral blood sample of 60 ml or bone marrow [11]. In addition, flow cytometry-based cell sorting devices are not available in every diagnostic laboratory. Therefore, we evaluated an automated magnetic cell-enrichment strategy for CD34+ cell selection from 20-ml peripheral blood, which is rapid, reliable, and does not depend on a flow cytometry cell-sorting facility. Our novel semi-automated method enables CD34+ DCC analysis in all hematological laboratories. As DCC of the CD34+ cell population out of peripheral blood can predict relapse earlier than white blood cell (WBC) DCC [7] our uncomplicated method provides the advantages of feasible engraftment and relapse monitoring in SCT patients with high sensitivity at low costs.
Patients and methods
Patients were treated and transplanted in the Department of Hematology, Oncology, and Immunology, University Hospital Giessen and Marburg, Campus Marburg. Peripheral blood samples were drawn after informed consent according to the demands of the local ethics committee. The study cohort consisted of 85 patients (male n = 47, female n = 38) with a median age of 48 years (18–68 years). Only patients with AML (n = 79) and MDS (n = 6) expressing the CD34 surface antigen on the main blast population at first diagnosis were enrolled in this study. The patients had received peripheral blood stem cell grafts from siblings (n = 27), matched related donors (n = 35) and mismatched unrelated donors (n = 23). Conditioning was performed myeloablative (n = 49) or in reduced intensity (n = 36) according to the definition of the EBMT [13].
Median duration of follow-up for all patients was 467 days (range 24–1,455 days) and median frequency of measurements was 6 (range 1–22) for CD34+ DCC and 5 (range 1–19) for WBC DCC. The disease entities and the risk groups of the whole study cohort are denoted in Table 1.
Magnetic CD34+ cell-enrichment
Twenty-milliliter peripheral blood was collected from each patient. Total WBC were recovered by dextran separation as follows: the blood was suspended 5:1 in a 5 % dextran (Sigma-Aldrich®, Munich, Germany) solution and allowed to sediment for 40 min at room temperature, before the leukocytes were removed from the upper phase and washed with phosphate buffered saline (PBS, Invitrogen™, Darmstadt, Germany). Remaining erythrocytes were lysed 5 min at room temperature in an ammonium chloride (Merck, Darmstadt, Germany) containing solution. Labeling of total white blood cells with ferromagnetic monoclonal CD34 antibodies was performed according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany). In brief, up to 2 × 108 cells were washed in PBS and incubated in 250-μl running buffer with 100-μl CD34 MicroBeads (clone QBEND/10) and 100-μl blocking reagent for 30 min at 4 °C protected from light. Afterwards, cells were washed with running buffer and separated with the autoMACS® Pro Separator (Miltenyi Biotec) in mode posseld2 (repeated magnetic separation). Up to six samples were run consecutively in this device and CD34+ cells were separated automatically.
Flow-cytometric CD34+ cell analysis and cell sorting
For CD34+ cell quantification and purity analyses, 100 μl of CD34+ enriched cell solution were incubated 20 min at room temperature with the fluorescent monoclonal antibodies CD34-PE (clone 8G12) and CD45-PerCp-Cy5.5™ (clone 2D; both from BD™ Biosciences, Heidelberg, Germany), washed and resuspended with PBS, followed by flow-cytometric analysis on a LSR2 device (BD™ Biosciences). The percentage of CD34+ cells was determined within the CD45+ leukocyte population. For dead cell exclusion 2-(4-amidinophenyl)-1H -indole-6-carboxamidine (DAPI, Sigma-Aldrich®) was added to a final concentration of 2 μg/ml. Subsequent data analysis was done with FLOWJO® software v.7.2.4 (TreeStar Inc., Ashland, OR, USA). One patient exhibited a decline of both total WBC and CD34+ donor cell chimerism. The peripheral blood of this patient was subjected to density-gradient centrifugation (Ficoll-Paque™ Plus, density 1.077 g/ml, GE Healthcare®, Freiburg, Germany) and mononuclear cells were stained with the following antibodies: CD3-FITC (clone SK7); CD56-PE (clone NCAM16.2) + CD16-PE (clone B73.1); CD19-APC (clone SJ25C1; all from BD™ Biosciences). The samples were resuspended in PBS buffer and sorted on a MoFlo® cell sorter device (Beckman Coulter, Krefeld, Germany). Genomic DNA was extracted from the sorted cell samples as described below.
DNA extraction and multiplex STR-profiling
Genomic DNA from the enriched CD34+ cells was extracted using the QIAamp® DNA Investigator Kit (QIAGEN®, Hilden, Germany) on the automated DNA preparation device QIAcube® (QIAGEN®) according to the manufacturer’s instructions. The final elution volume was 30 μl of distilled water.
Then, 1.5-ng DNA was used for amplifying 12 STR loci and the amelogenin locus by PCR, generating a STR profile with the Humantype Chimera® Kit (Biotype®, Dresden, Germany). The PCR-amplified STR fragments were separated by capillary electrophoresis on the ABI Prism® 310 Genetic Analyzer (Applied Biosystems®, Foster City, CA, USA). Data were analyzed with the GeneMapper v.3.7 (Applied Biosystems®) software and donor chimerism was calculated employing ChimeraSoft v.1.05 (Biotype®) software.
Results
Functionality of the semi-automated CD34+ donor cell chimerism analysis assay
CD34+ cells occur in the peripheral blood in a very low percentage (0.01–0.2 %). Thus, efficient and reliable cell-enrichment strategies are mandatory prior to further analysis of genomic DNA. Median purity of CD34+ cells after semi-automated enrichment as determined by flow-cytometric analysis was 85 % (range 83.5–86.5 %). Despite the low absolute number of total CD34+ cells obtained from 20-ml peripheral blood, our assay provided about 500 ng DNA on average (range 240 to 1,020 ng in 20 selected samples). Even with a total leukocyte count ranging between 0.1 and 1/nl during the early post-transplantation period, we achieved an adequate yield of genomic DNA from semi-automatically enriched CD34+ cells.
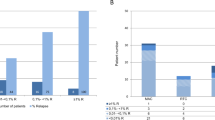
In order to determine the normal range for CD34+ DCC and WBC DCC, we summarized 668 values from 57 patients of the study cohort that proved to have no molecular or cytological relapse in the follow-up period. These reference DCC values are depicted in Fig. 1. Average CD34+ DCC (n = 394) was slightly lower than for WBC DCC (n = 274) with a median of 97 % (range 84–100 %, standard deviation 2.59) and 98 % (range 94–100 %, standard deviation 1.26), respectively; 2.23 % (9/394) of the CD34+ DCC reference values ranged between 84 and 90 %.
Normal ranges of CD34+ and WBC DCC values in multiplex STR-PCR analysis from 57 patients without molecular or cytological relapse. In total, 394 CD34+ DCC values and 274 WBC DCC values were depicted. CD34+ DCC values ranged from 84 to 100 % (median 97 %, standard deviation 2.59) and WBC DCC values ranged from 94 to 100 % (median 98 %, standard deviation 1.26)
CD34+ DCC never declined below 84 % with our semi-automated assay except in relapsed situations and in one case of presumably autologous hematopoietic regeneration. Thus, the cut-off value of CD34+ DCC was set to 80 % as already suggested for flow-cytometric cell sorting-based analyses of CD34+ DCC and cut-off value of WBC DCC was set to 94 % [7].
Semi-automated CD34+ donor cell chimerism analysis can predict forthcoming relapse
CD34+ DCC monitoring in transplanted AML and MDS patients provides the opportunity to detect imminent relapse particularly early, i.e., 12–97 days (median 52 days) before the clinical diagnosis of relapse as reported by Thiede et al. [11].
In our patient cohort, relapse occurred in 24 cases. From 22 patients with relapse, 2 patients (unique patient number (UPN) 25 and 68, Table 2) suffered a second relapse after the second allogeneic stem cell transplantation within the observation period. CD34+ DCC had dropped below 80 % in all patients, except in one patient with isolated cerebral relapse. This indicates an assay sensitivity of 100 % for systemic relapse situations. AML relapses within the central nervous system are apparently not detectable with this method. Concomitant WBC DCC had decreased below 94 % in 17/22 cases of relapse. The characteristics of patients with relapse are denoted in Table 2.
Applying CD34+ DCC, relapse was detected significantly better as compared to the conventional WBC DCC in a time interval of 8–56 days before cytological relapse occurred (p = 0.043, Fisher’s exact test). In our analyses, CD34+ DCC declined <80 % in a time interval between 29–42 days before cytological relapse. WBC DCC declined <94 % in a period of 0–7 days before or at cytological relapse. Analyses are depicted in Fig. 2.
Decrease of CD34+ DCC indicated imminent relapse earlier than WBC DCC. CD34+ DCC analysis was significantly better for detection of relapse at the time interval 8–56 days prior to cytological relapse (p = 0.043, Fisher’s exact test). CD34+ DCC declined <80 % in a time interval between 29–42 days before cytological relapse. WBC DCC declined <94 % in a period of 0–7 days before or at cytological relapse. The black lines are indicating the median for each time interval. The amount of CD34+ and WBC DCC measurements for each time interval was n = 9/7 for days 43–56; n = 7/4 for days 29–42; n = 8/7 for days 15–28; n = 5/4 for days 8–14; and n = 19/19 for days 0–7
AML patient (UPN 2) presented with a decline of the CD34+ DCC 1,343 days after allogeneic SCT down to 86 % and 23 days later to 77 %. Bone marrow aspiration was performed and subsequent cytology showed no evidence of relapse at that point in time. But cytogenetic analyses from bone marrow aspirate detected the upcoming leukemia (46XX t(4;19)(q24;q13)[5], 46XY[15]). The patient was treated with five doses of donor T cells at increasing dosages until the CD34+ DCC value increased to 98 %. Four hundred forty days after the first decline of CD34+ DCC, patient UPN 2 suffered overt relapse and died 1,834 days after allogeneic SCT because of a sepsis during reinduction chemotherapy. The early therapeutic intervention delayed full-blown relapse in this patient for 14 months.
We conclude that our assay is equal to the previously described time-consuming flow-cytometric based enrichment method [11] and detects relapse significantly earlier compared to WBC DCC.
Semi-automated CD34+ DCC is useful for monitoring of engraftment
Applying our novel CD34+ DCC method to an AML patient (UPN 58) who suffered from overt relapse at day 320 post-transplantation, the CD34+ DCC was determined as 36 % while the corresponding WBC DCC value remained 92 % (Fig. 3). Two cycles of low-dose Ara-C and four donor lymphocyte infusions were administered to the patient, but the CD34+ DCC value dropped even further to less than 10 %. Subsequently, the patient received high-dose Ara-C (5 × 3 g/m2 body surface area, BSA) day 1–5 and received a second SCT from the same matched unrelated donor (conditioning regimen: fludarabine 5 × 30 mg/m2 BSA, melphalan 150 mg/m2 BSA, and antithymocyte globulin from rabbit (Fresenius Biotech, Munich, Germany) 4 × 10 mg/kg bodyweight, BW).
Monitoring efficacy of donor lymphocyte infusion (DLI) and Ara-C (light gray bar) treatment after relapse. WBC and CD34+ DCC values were measured in AML patient UPN 48 who suffered from relapse 328 days after allogeneic SCT. At the time of relapse WBC and CD34+ DCC were determined as 92 and 36 %, respectively. Two cycles of low-dose Ara-C and four doses of DLI were administered to the patient. High-dose (HD) Ara-C, followed by a second SCT lead to increase of both WBC and CD34+ DCC in this patient. Forty-two days after the second SCT, WBC and CD34+ DCC values were determined as 98 and 97 %, thus reflecting successful engraftment in this patient
Forty-two days after the second SCT, WBC and CD34+ DCC were determined as 98 and 97 %, respectively. The patient is still in complete remission and alive until the end of follow-up. This time-course demonstrates that the CD34+ DCC is superior in reflecting the relapse and engraftment situations of patients. In summary, our semi-automated CD34+ DCC not only predicts relapse better than total WBC DCC but is also useful for the accurate and informative monitoring of stem cell engraftment.
Autologous recovery 2 years after allogeneic stem cell transplantation is a rare cause for a decline of CD34+ donor cell chimerism
According to our experience with semi-automated CD34+ DCC for monitoring 85 allogeneic SCT patients relapse was the only condition that induced a decline in CD34+ DCC below 80 % except for one case. In this AML patient (UPN 45), the WBC and CD34+ DCC both decreased 2.5 years after allogeneic SCT from a matched unrelated donor (Fig. 4a). Relapse was suspected and bone marrow aspiration was performed. There were no cytological signs for a relapse and treatment-related MDS detectable, neither in the bone marrow nor in the peripheral blood. The patient was otherwise healthy without any apparent sign of relapse or severe chronic graft-versus-host disease (GvHD). In subsequent analyses, WBC and CD34+ DCC values both declined to a minimum of 58 % at day 1,128 post-transplantation. In order to understand the discrepancy of these findings, we performed DCC of different blood cell subsets. T and B lymphocytes and NK cells showed an almost complete donor profile, while CD34+ cells, monocytes, and granulocytes revealed a donor cell signal of 60–67 % (Fig. 4b). From these results, we deduced that a healthy myeloid precursor cell clone had survived the initial induction chemotherapy and the condition regimen (fludarabine 4 × 30 mg/m2 BSA, Ara-C 4 × 2 g/m2 BSA, 4-Gy total body irradiation, cyclophosphamide 2 × 40 mg/kg BW and antithymocyte globulin from rabbit (Genzyme Cambridge, MA, USA) 3 × 2.5 mg/kg BW), now expanding in the absence of GvHD. After administration of five DLI in increasing dosages (1 × 105, 5 × 105, 1 × 106, 5 × 106, 8 × 106 CD3+ T cells/kg BW) mild GvHD was induced. The WBC and CD34+ DCC increased to >90 %. There were no signs for disease relapse in this patient until end of follow-up. Taken together, autologous regeneration after allogeneic SCT can be a rare cause for decline in CD34+ and WBC DCC.
Recovery of healthy autologous stem cells after allogeneic transplantation caused a decline of CD34+ donor cell chimerism (a). In AML patient UPN 45, WBC and CD34+ DCC both dropped to 80 % 2.5 years after SCT without any sign of relapse. After donor lymphocyte infusions (DLI) both WBC and CD34+ DCC values increased to 98 %. Donor cell chimerism was determined in different cellular compartments (b). T, NK, and B cells exhibited a complete donor profile while monocytes and granulocytes showed a significant decrease of donor cell chimerism
Discussion
Measuring CD34+ DCC from the peripheral blood is superior to WBC DCC for detection of imminent relapse in patients with CD34+ acute myeloid leukemia and myelodysplastic syndrome receiving allogeneic stem cell transplantation [7].
We established a semi-automated CD34+ DCC assay combining the advantages of a sensitive disease monitoring with a rapid standardized operation procedure at low costs. This makes our approach appropriate for the hematologic laboratories of transplantation clinics.
A complementary report from Scheffold et al. demonstrated that a decrease of marrow CD34+ donor cell chimerism was highly predictive for relapse in AML patients with a CD34+ leukemic phenotype [12]. However, instead of bone marrow aspirates, only 20 ml of peripheral blood were required for our assay without the need for repeated marrow punctures every 4 weeks [12]. Our novel method was applicable even in the early post-transplantation period with a WBC count of <1/nl. Sufficient amounts of DNA and thus clear and significant STR profiles have always been obtained. A decline of CD34+ DCC below 80 % was found to be highly sensitive (100 %) and predictive of imminent systemic relapse, similar to the peripheral blood CD34+ DCC method reported before [7]. Likewise, we recommend a cut-off value of 80 % for our semi-automated CD34+ DCC assay. Autologous regeneration of host stem cells after allogeneic SCT has to be considered as a rare differential diagnosis if CD34+ DCC values decline to 80 %.
Options and treatment strategies for relapse situations after allogeneic SCT are currently limited. Immunomodulatory strategies, donor lymphocyte infusions, or a second SCT have been applied with limited success [17–19]. Efficacy for low-dose azacytidine treatment in combination with DLI in AML patients who relapsed after allogeneic SCT has been demonstrated recently by Lubbert et al. [20]. First promising responses in patients with a decline of CD34+ DCC have been reported recently upon treatment with four azacytidine cycles (75 mg/m2/day for seven consecutive days) [9]. In this study, 50 % (10/20) of patients had a major response, i.e., they became MRD negative, and four of these patients sustained a MRD negative status for 1 year of follow-up. Azacytidine does not only exhibit cytotoxicity against leukemic blasts, but has recently been shown to enhance the number of regulatory T cells and therefore augment graft-versus-leukemia effect in patients [21]. These are promising data that emphasize the clinical value of a sensitive MRD monitoring with CD34+ DCC after SCT, in particular when PCR-detectable disease-specific gene products are missing. With the upcoming novel-targeted drug therapies like the tyrosine kinase inhibitor sorafenib, remissions can be achieved even in the case of relapse after SCT [22]. AML patients who harbor a FLT3-ITD mutation and receive sorafenib for relapse therapy after allogeneic SCT benefit even more from sorafenib treatment compared to primary AML patients in the first relapse [23]. Thus, our novel and feasible CD34+ donor chimerism analysis method paves the way for further studies with early intervention strategies for imminent relapse of AML after SCT.
References
Cornelissen JJ, van Putten WL, Verdonck LF et al (2007) Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood 109:3658–3666
Koreth J, Schlenk R, Kopecky KJ et al (2009) Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 301:2349–2361
Chang C, Storer BE, Scott BL et al (2007) Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood 110:1379–1387
Kern W, Haferlach C, Haferlach T et al (2008) Monitoring of minimal residual disease in acute myeloid leukemia. Cancer 112:4–16
Thiede C, Bornhauser M, Oelschlagel U et al (2001) Sequential monitoring of chimerism and detection of minimal residual disease after allogeneic blood stem cell transplantation (BSCT) using multiplex PCR amplification of short tandem repeat-markers. Leukemia 15:293–302
Lange T, Hubmann M, Burkhardt R et al (2011) Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia 25:498–505
Bornhauser M, Oelschlaegel U, Platzbecker U et al (2009) Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica 94:1613–1617
Schmid C, Schleuning M, Aschan J et al (2004) Low-dose ARAC, donor cells, and GM-CSF for treatment of recurrent acute myeloid leukemia after allogeneic stem cell transplantation. Leukemia 18:1430–1433
Platzbecker U, Wermke M, Radke J et al (2011) Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia 26(3):381–389
Bader P, Willasch A, Klingebiel T (2008) Monitoring of post-transplant remission of childhood malignancies: is there a standard? Bone Marrow Transplant 42(Suppl 2):S31–S34
Thiede C, Lutterbeck K, Oelschlagel U et al (2002) Detection of relapse by sequential monitoring of chimerism in circulating CD34+ cells. Ann Hematol 81(Suppl 2):S27–S28
Scheffold C, Kroeger M, Zuehlsdorf M et al (2004) Prediction of relapse of acute myeloid leukemia in allogeneic transplant recipients by marrow CD34+ donor cell chimerism analysis. Leukemia 18:2048–2050
Martino R, Iacobelli S, Brand R et al (2006) Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood 108:836–846
Döhner H, Estey EH, Amadori S et al (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115:453–474
Swerdlow SH, Campo E, Harris NL et al (2008) In: Campo E (ed) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon
Greenberg P, Cox C, LeBeau MM et al (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079–2088
Levine JE, Braun T, Penza SL et al (2002) Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol 20:405–412
Collins RH Jr, Shpilberg O, Drobyski WR et al (1997) Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 15:433–444
Eapen M, Giralt SA, Horowitz MM et al (2004) Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant 34:721–727
Lubbert M, Bertz H, Wasch R et al (2010) Efficacy of a 3-day, low-dose treatment with 5-azacytidine followed by donor lymphocyte infusions in older patients with acute myeloid leukemia or chronic myelomonocytic leukemia relapsed after allografting. Bone Marrow Transplant 45:627–632
Goodyear OC, Dennis M, Jilani NY et al (2012) Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 119:3361–3369
Metzelder S, Wang Y, Wollmer E et al (2009) Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood 113:6567–6571
Metzelder SK, Schroeder T, Finck A et al (2012) High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia 26(11):2353–2359
Acknowledgments
We would like to thank Christiane Rohrbach for technical assistance and the Flow Cytometry Core Facility Marburg for their kind support. This work was funded by “Kooperationsvertrag §2 Abs. 3 Universitätsklinikum Giessen und Marburg GmbH (UKGM)” and the José Carreras Leukemia Foundation (grant AH06-01, to AN). Our work was also supported by Alfred and Ursula Kulemann Stiftung and the German Research Council “Klinische Forschergruppe KFO 210” (to AB, AN, CB).
Conflicts of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoffmann, J.C., Stabla, K., Burchert, A. et al. Monitoring of acute myeloid leukemia patients after allogeneic stem cell transplantation employing semi-automated CD34+ donor cell chimerism analysis. Ann Hematol 93, 279–285 (2014). https://doi.org/10.1007/s00277-013-1961-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1961-4