Abstract
The aim of this study was to evaluate the impact of the response to induction therapy on the long-term prognosis of multiple myeloma (MM) after autologous stem cell transplantation (ASCT) in the era of novel agents (NAs). A total of 171 patients who were newly diagnosed with MM and underwent early ASCT were analyzed. One hundred ten had a NA-based induction therapy, and 61 patients had a non-NA-based induction therapy. After a median follow-up of 45.4 months, the 4-year overall survival (OS) and progression-free survival (PFS) from transplantation were 60.5 and 25.5 %, respectively, for the NA-based induction group and 54.6 and 15.6 %, respectively, for the non-NA-based induction group. Multivariate analyses revealed that the patients who had NA-based induction had a significantly shorter OS (P < 0.001) and PFS (P < 0.001) when at least a partial response (PR) was not achieved. In patients who did not receive NAs before ASCT, lack of at least a PR to induction therapy was not associated with a survival disadvantage. These findings suggest that, unlike pretransplantation induction before NAs, patients who do not respond to induction treatment using NAs may not derive a benefit from ASCT. The relevance of induction failure differs for corticosteroid- and NA-based induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-dose therapy followed by autologous stem cell transplantation (ASCT) is the platform for transplant-eligible patients with multiple myeloma (MM). Previous reports showed that ASCT for patients with MM who did not achieve at least a partial response (PR) to pretransplant induction therapies did not have a compromised survival outcome [1, 2]. All of these data, however, predate the introduction of novel agents (NAs).
Gertz et al. evaluated the effect of response to induction therapy on prognosis in patients with MM who underwent ASCT after thalidomide- or lenalidomide-based induction therapy. A <50 % decrease in serum M-protein predicted worse overall survival (OS) and progression-free survival (PFS) after ASCT [3]. This result contrasts results before NAs were introduced, in which responses to pretransplant induction therapies were not associated with long-term outcomes [1, 2]. Recently, Awan et al. reported conflicting results on the effect of response to NAs on ASCT outcomes. They analyzed 127 patients with MM who were receiving NAs as induction therapy and found that patients with <PR (n = 17) did not have a poorer OS or PFS, than patients with ≥PR (n = 110) [4]. Therefore, the effect of response to NA-based induction therapies, including proteasome inhibitors and immunomodulatory drugs, on long-term prognosis remains elusive.
To address this issue, we stratified patients according to the response to induction with NAs containing bortezomib and/or thalidomide and non-NA induction therapies, and investigated the long-term outcomes after ASCT. Additionally, we compared detailed response groups, including complete response (CR), very good PR (VGPR), PR, and stable disease (SD). Data were analyzed to allow for adequate long-term follow-up given the potentially slow rate of progression in MM.
Materials and methods
Patient selection
Between February 1999 and July 2010, 248 adults newly diagnosed with MM at the Catholic Blood and Marrow Transplantation Center received early ASCT, defined as transplantation within 1 year of initial diagnosis and as part of first-line therapy, following NA-based or non-NA-based induction chemotherapy. Among these patients, 77 were not eligible because of tandem transplants (n = 73) or early transplant-related mortalities (n = 4); thus, 171 patients were evaluated in this study. Patients who had progressive disease before ASCT did not proceed to transplantation. The treatment schedule is summarized in Fig. 1. The Institutional Review Board of The Catholic University of Korea approved the research protocol for data analysis, and this study was conducted in accordance with the Declaration of Helsinki.
Treatment regimens and transplant procedures
NA-based induction chemotherapy consisted of bortezomib ± dexamethasone (n = 87), bortezomib + dexamethasone + thalidomide (n = 14), or thalidomide + dexamethasone (n = 9). Sixty-one patients received non-NA-based induction chemotherapy, including high-dose dexamethasone or vincristine, adriamycin, and dexamethasone (VAD). The treatment protocols for both groups (NA-based group vs non-NA-based group) were the same except for the induction chemotherapy. General ASCT procedures were performed as described in previous reports [5]. Briefly, all patients were mobilized with cyclophosphamide (3 g/m2 total) over 2 days followed by G-CSF (lenograstim, JW Pharmaceutical, Seoul, Korea) at 10 μg/kg/day, subcutaneously once a day. Conditioning consisted of melphalan (100 mg/m2) for 2 days. G-CSF (5 μg/kg/day) was administered subcutaneously to all patients from 1 day after transplantation until the absolute neutrophil count (ANC) was >3.0 × 109/L. All patients received prophylactic antibiotics and an antifungal agent (fluconazole) starting 4 days before transplantation until the ANC was 1.5 × 109/L.
Definitions and evaluation of response
Stage was classified by the Durie–Salmon staging system, and treatment response was assessed according to the criteria from the International Myeloma Working Group [6]. Using the response to induction chemotherapy, we stratified each group of patients into four subgroups: (1) CR, (2) VGPR, (3) PR, and (4) SD. OS from transplantation was defined as the time from ASCT to death from any cause, and surviving patients were censored at the last follow-up. PFS was measured as the time from ASCT to disease progression or death (regardless of cause), whichever comes first. Time to progression (TTP) was calculated as time from ASCT to disease progression, with deaths due to causes other than progression censored.
Statistical analysis
Our main objectives were to evaluate the probability of PFS and OS from the date of ASCT and to measure TTP. The probabilities of PFS and OS after ASCT were plotted using the Kaplan–Meier method. Potential prognostic factors for PFS and OS were assessed using a two-tailed log-rank test in each induction chemotherapy group, including age, sex, stage at diagnosis, serum M-protein type, myeloma bone disease apparent on plain radiographs at diagnosis, hemoglobin level, serum LDH, creatinine, calcium, β2-microglobulin, albumin levels, and percentage of bone marrow plasma cells. The number of patients having cytogenetic data was too small to provide meaningful information, and hence, their data were not included. Covariates having a P value of less than 0.1 in the univariate analyses were added to a Cox proportional hazards regression model, in which all P values were two-sided, and statistical significance was set at P < 0.05. The association between the categorical variables was assessed using either χ 2 or Fisher’s exact tests. The Mann–Whitney U test was used to compare continuous variables.
Results
Patient characteristics
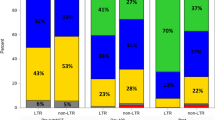
A total of 171 MM patients were included in this study, of whom 90 (53 %) were male and 81 (47 %) were female. The median age was 53 years (range, 34–65 years), and the median disease duration before ASCT was 7.0 months (range, 3.3–11.0 months). Stage IIA, IIIA, and IIIB diseases at diagnosis comprised 9, 74, and 17 % of subjects, respectively. Table 1 lists the demographic information for all patients. After induction chemotherapy, in the NA-based induction group, 42 (38 %), 18 (16 %), and 40 (36 %) patients had a CR, VGPR, and PR, respectively, while 10 (9 %) patients had SD. In the non-NA-based group, 9 (15 %), 8 (13 %), 33 (54 %), and 11 (18 %) patients had a CR, VGPR, PR, and SD after induction therapy, respectively. Evaluation of the CR status did not require a bone marrow examination. There was a trend toward higher PR or better response after NA-based induction therapy (91 %) than after non-NA-based induction therapy (82 %) (P = 0.088).
Overall ASCT outcomes
The median follow-up was 45.4 months (range, 38.3–52.4 months) for survivors. A total of 35 (32 %) patients in the NA-based induction group and 44 (72 %) in the non-NA-based induction group died. In addition, 68 (62 %) patients in the NA-based induction group and 50 (82 %) in the non-NA-based induction group had disease progression. For the NA and non-NA groups, the 4-year OS was 60.5 and 54.6 %, respectively (median 67.7 and 52.2 months; P = 0.145), and the 4-year PFS was 25.5 and 15.6 %, respectively (median 24.0 and 36.9 months; P = 0.656). TTP for the NA and non-NA groups had a median of 24.0 (95 % CI, 17.6–30.4) and 27.1 (95 % CI, 23.6–30.7) months from ASCT, respectively, which were not significantly different (P = 0.689).
Influence of response to pretransplant induction therapy on long-term outcomes after ASCT
To assess the impact of the response to pretransplant induction therapy, outcomes of patients were analyzed according to the depth of response (CR + VGPR vs PR vs <PR) on the basis of the type of induction therapy (Fig. 2). In the NA-based group, the 4-year OS of patients with CR + VGPR, PR, and <PR was 62.2, 67.4, and 10.0 % (median OS not reached vs 67.7 vs 11.6 months; P < 0.001), respectively, and the 4-year PFS was 30.2, 25.9, and 0 % (median OS 26.6 vs 19.1 vs 4.7 months; P < 0.001), respectively. In contrast, in the non-NA-based group, there was no difference of OS according to response (median OS 71.8 vs 52.1 vs 45.5 months; P = 0.152). Regarding 4-year PFS, the patients with CR + VGPR, PR, and <PR showed 39.4, 9.6, and 0 % (median PFS 32.7 vs 23.5 vs 24.6 months; P = 0.024), respectively.
We also compared the outcomes of patients by more detailed responses. In the NA-based group, the median OS was not reached for the CR and VGPR subgroups and was 67.7 and 11.6 months for the PR and SD subgroups, respectively. The median PFS was 30.0, 24.0, 19.1, and 4.7 months for the CR, VGPR, PR, and SD subgroups, respectively, after induction therapy. In the non-NA-based group, the median OS was 60.1, 83.7, 52.1, and 45.5 months, and the median PFS was 32.7, 56.8, 23.5, and 26.9 months for the CR, VGPR, PR, and SD subgroups, respectively, after induction therapy (Online resource Fig. 1).
Multivariate analysis of prognostic factors affecting long-term outcomes after ASCT
Of factors that affected survival outcomes in the NA-based group on univariate analyses, multivariate analyses showed that patients with <PR had a lower OS (RR of 6.46, P < 0.001) and PFS (RR of 6.64, P < 0.001) than patients with CR + VGPR (Table 2), whereas there was no difference between CR + VGPR and PR for OS (RR of 1.16, P = 0.796) and PFS (RR of 1.22, P = 0.497). Additionally, higher serum β2-microglobulin level and myeloma bone disease on plain radiographs at diagnosis were associated with poor PFS.
Of factors affecting survival outcomes in the non-NA-based group on univariate analyses, multivariate analyses showed that no survival advantage was observed according to responses to pretransplant induction therapy (Table 2). However, the <PR subgroup tended to have a poor PFS (RR of 2.38, P = 0.072). Additionally, higher serum creatinine and posttransplant non-CR were predictors of poor OS. Posttransplant non-CR also tended to have a poor PFS.
Multivariate analyses, including detailed pretransplant responses (CR, VGPR, PR, and SD), are presented in Online resource Table 1. In the NA-based group, patients achieving a VGPR or PR had a similar OS and PFS to patients achieving a CR, while those with SD had a significantly shorter OS and PFS compared to those achieving a CR. In the non-NA-based group, however, any detailed response to induction therapy did not predict long-term outcomes.
Discussion
Since there has been a large change in the initial therapy of MM during the past few years with early incorporation of NAs, this study emphasizes the importance of different responses to induction therapy with NAs and non-NAs to predict survival outcomes after ASCT. To refine patient selection for ASCT according to the response to induction treatment, we evaluated a group of patients who had either NA-based (bortezomib and/or thalidomide) or corticosteroid-based induction. We then classified patients according to the depth of response before administering high-dose therapy on the basis of the type of induction therapy. Among the patients treated with NAs before ASCT, the response was an independent prognostic factor, whereas among patients treated with corticosteroid-based induction, the response did not have any long-term implications.
Before the era of NAs, transplant-eligible patients with MM who do not achieve an objective response to induction therapy have never been shown to have inferior outcomes following ASCT when compared to patients with chemosensitive disease. Therefore, high-dose therapy has been administered whether or not the disease responded to induction therapy without compromising PFS or OS [2, 7–9]. In the past decade, however, highly effective NAs have become available and have been used for initial therapy in patients newly diagnosed with MM [10–12]. Incorporating new drugs into the initial treatment has resulted in higher response rates, including CR rates, than those seen with previous steroid-based regimens. Compared to dexamethasone or VAD, combined thalidomide and dexamethasone as induction treatment increased the overall response rate but failed to increase the CR rate before transplantation or the CR plus VGPR rate after ASCT [13]. A randomized trial showed that, compared with VAD, bortezomib plus dexamethasone significantly improved CR plus VGPR before ASCT [14]. Recently, the most promising results have been obtained with a three-drug regimen consisting of thalidomide, bortezomib, and dexamethasone [15]. In the current study, however, we did not observe any differences between NA-based and non-NA-based induction groups in terms of PFS, OS, or TTP despite a more high-quality response before ASCT in the NA-based induction group. The short duration of therapy before ASCT with any of these agents probably does not have a long-term effect on survival outcomes after transplantation. However, given that patients not receiving NAs as pretransplant treatment appeared to have higher ≥PR rates (82 %), this result may be biased to some extent by retrospectively evaluating treatment responses. Other possible explanations may be different follow-up periods between the NA-based induction group and the non-NA-based induction group (P < 0.007), and administration of NAs after relapse may be more effective in the patients without NA exposure. A meta-analysis of randomized, controlled trials of NA-based regimens as induction treatment before ASCT in newly diagnosed MM showed improved CR and PFS but not OS [16]. In their analyses, the potential survival benefits of the NA-based induction were observed in a long-term follow-up study. Further intensification of the induction regimen using NAs has been shown to improve response rates before ASCT, but the impact on OS has not been established [17].
The current study found an important relationship between the survival outcomes and the respective responsiveness to either NAs or conventional induction regimens. A poor response to dexamethasone or VAD did not appear to alter the natural course of the disease. Given that a failed response to induction with NAs correlated with a reduced benefit from ASCT, response to NAs may be a surrogate marker for biological characteristics that would improve long-term outcomes. These data also were supported by the findings of previous studies. Gertz et al. evaluated the impact of response failure with thalidomide or lenalidomide induction on survival outcomes after ASCT and found that unlike the non-NA era, patients who do not achieve PR have a significantly shorter OS and PFS [3]. Our results could be related to selection bias because a subgroup of patients with failure of non-NA induction therapy may include some patients who were sensitive to novel salvage therapy. A disease that does not respond to NAs before ASCT has increased kinetic activity with more rapid regrowth after cytoreduction with high-dose melphalan, resulting in short PFS. Patients with disease that did not respond to NAs at induction could not be salvaged with NAs on progression, resulting in short OS. Awan et al., however, demonstrated that high-dose melphalan remains one of the most active therapies for patients with MM, and a failed response to novel induction regimens should not automatically preclude consideration of ASCT in patients with refractory disease [4]. Prospective studies are required to prove whether MM patients who are refractory to novel induction therapies should not routinely be considered ineligible for ASCT.
The results of the current study should be used in a limited manner to provide novel therapies in the context of ASCT. One caveat of this retrospective study is that heterogenous NA-based combinations were used as an induction therapy in the NA-based group. It is not obvious that any type of NA (bortezomib or thalidomide) treatment mainly contributes to the resistance to high-dose melphalan. Patients who received induction treatment with NAs showed good response to NAs, and the proportion of those with <PR was relatively small. The type of induction treatment is determined on the basis of coverage by the National Health Insurance Program in South Korea. It does not cover the costs of some NAs as frontline treatment, and the selection of induction regimen has been diverse. Induction treatment was performed at the physician’s discretion for some patients who had been transferred to our institute from other hospitals to receive ASCT. To minimize the bias, we focused on a relatively homogeneous population, consecutive patients with newly diagnosed MM who received early ASCT (1 year of initial diagnosis and as part of first-line therapy).
In conclusion, although interpretation with caution is needed because of the limitations of a small number of patients, our results show that patients who do not respond to induction with NAs do not benefit from ASCT, suggesting that the relevance of induction failure depends on whether the induction is a NA-based or conventional treatment. Patients with a suboptimal response to induction with NAs could be offered an alternative therapy. However, a larger study should be performed to verify that a response to NAs, or lack thereof, precludes ASCT.
References
Kumar S, Lacy MQ, Dispenzieri A, Rajkumar SV, Fonseca R, Geyer S, Allmer C, Witzig TE, Lust JA, Greipp PR, Kyle RA, Litzow MR, Gertz MA (2004) High-dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Bone Marrow Transplant 34:161–167
Singhal S, Powles R, Sirohi B, Treleaven J, Kulkarni S, Mehta J (2002) Response to induction chemotherapy is not essential to obtain survival benefit from high-dose melphalan and autotransplantation in myeloma. Bone Marrow Transplant 30:673–679
Gertz MA, Kumar S, Lacy MQ, Dispenzieri A, Dingli D, Hayman SR, Buadi FK, Hogan WJ (2010) Stem cell transplantation in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood 115:2348–2353
Awan FT, Osman S, Kochuparambil ST, Gibson L, Remick SC, Abraham J, Craig M, Jillella A, Hamadani M (2012) Impact of response to thalidomide-, lenalidomide- or bortezomib-containing induction therapy on the outcomes of multiple myeloma patients undergoing autologous transplantation. Bone Marrow Transplant 47:146–148
Lee SE, Yahng SA, Cho BS, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, Kim DW, Lee JW, Min WS, Park CW, Min CK (2012) Lymphocyte subset analysis for the assessment of treatment-related complications after autologous stem cell transplantation in multiple myeloma. Cytotherapy 14:505–512
Kyle RA, Rajkumar SV (2009) Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23:3–9
Alexanian R, Dimopoulos MA, Delasalle KB, Hester J, Champlin R (1995) Myeloablative therapy for primary resistant multiple myeloma. Stem Cells 13(Suppl 2):118–121
Barlogie B, Alexanian R, Dicke KA, Zagars G, Spitzer G, Jagannath S, Horwitz L (1987) High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood 70:869–872
Kumar SK, Dingli D, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Rajkumar SV, Litzow MR, Gertz MA (2008) Impact of pretransplant therapy in patients with newly diagnosed myeloma undergoing autologous SCT. Bone Marrow Transplant 41:1013–1019
Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR, Eastern Cooperative Oncology G (2006) Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol 24:431–436
Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J (2005) Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol 129:776–783
Lacy MQ, Gertz MA, Dispenzieri A, Hayman SR, Geyer S, Kabat B, Zeldenrust SR, Kumar S, Greipp PR, Fonseca R, Lust JA, Russell SJ, Kyle RA, Witzig TE, Bergsagel PL, Stewart AK, Rajkumar SV (2007) Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clinic Proc 82:1179–1184
Cavo M, Zamagni E, Tosi P, Tacchetti P, Cellini C, Cangini D, de Vivo A, Testoni N, Nicci C, Terragna C, Grafone T, Perrone G, Ceccolini M, Tura S, Baccarani M, Bologna 2002 Study et al (2005) Superiority of thalidomide and dexamethasone over vincristine-doxorubicindexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood 106:35–39
Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, Lenain P, Hulin C, Facon T, Casassus P, Michallet M, Maisonneuve H, Benboubker L, Maloisel F, Petillon MO, Webb I, Mathiot C, Moreau P (2010) Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005–01 phase III trial. J Clin Oncol 28:4621–4629
Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, Di Raimondo F, Crippa C, Zamagni E, Palumbo A, Offidani M, Corradini P, Narni F, Spadano A, Pescosta N, Deliliers GL, Ledda A, Cellini C, Caravita T, Tosi P, Baccarani M, Network GIM (2010) Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet 376:2075–2085
Wang L, Ran X, Wang B, Sheng Z, Liu L (2012) Novel agents-based regimens as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis of randomized controlled trials. Hematol Oncol 30:57–61
Giralt S (2011) Stem cell transplantation for multiple myeloma: current and future status. ASH Educ Program Book 1:191–196
Acknowledgments
The authors acknowledge all members of the Catholic Blood and Marrow Transplantation Center, particularly the house staff, for their excellent care of the patients. This study was supported by the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant no. HIA130000).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online resource Figure 1
Long-term outcomes according to detailed pretransplant responses. In the NA-based group, (A) overall survival and (B) progression-free survival. In the non-NA-based group, (C) overall survival and (D) progression-free survival (JPEG 174 kb)
Table 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Lee, SE., Yoon, JH., Shin, SH. et al. Impact of failed response to novel agent induction in autologous stem cell transplantation for multiple myeloma. Ann Hematol 93, 627–634 (2014). https://doi.org/10.1007/s00277-013-1911-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1911-1