Abstract
The frequency of breakthrough invasive fungal diseases (IFDs) during echinocandin therapy is unclear. We retrospectively analyzed 534 hematologic patients treated with echinocandin (caspofungin, N = 55; micafungin, N = 306; anidulafungin, N = 173). Four proven IFDs were found, caused by Candida parapsilosis (N = 2), C. parapsilosis and Candida glabrata (N = 1), and Fusarium species (N = 1). Four cases of possible IFDs were observed, all showing pulmonary infection. One case showed features suggestive of hepatosplenic candidiasis. Six of these eight cases had previously received the purine analog clofarabine. Breakthrough IFD during echinocandin treatment occurred infrequently (1.5 %), caused predominantly by Candida species. Clofarabine usage was an important risk factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The echinocandins are a new group of antifungal medications [1]. They inhibit the enzyme 1,3-β-d-glucan synthase, preventing the synthesis of 1,3-β-d-glucan that is an integral component of the cell wall of Candida and Aspergillus species [1]. Three echinocandins are available. Caspofungin is efficacious as empirical therapy of febrile neutropenia and has been evaluated for salvage treatment of invasive aspergillosis [1]. Micafungin is used as prophylaxis after hematopoietic stem cell transplantation (HSCT) [1]. Anidulafungin is approved for the treatment of esophageal candidiasis [1].
As data on echinocandins usage accumulate, breakthrough invasive fungal diseases (IFDs) are increasingly reported [2]. However, because of marketing strategies, few countries have all three echinocandins available at the same time. Therefore, pooling of data from different countries where the three echinocandins are not simultaneously available [2] might not reflect the actual frequencies and spectra of breakthrough IFD.
In order to define the frequency and clinical significance of breakthrough IFD during echinocandin therapy, we retrospectively analyzed a cohort of hospitalized hematologic patients treated with caspofungin, micafungin, and anidulafungin during a 42-month period.
Materials and method
Patients
The study was conducted in a quaternary hematologic referral center. Case files of all hematologic and HSCT patients who had received antifungal drugs from January 2009 to June 2012 were retrieved. Patients who had received echinocandins were reviewed. The choice of echinocandin was determined by the attending physicians. All records were scrutinized for breakthrough IFD.
Definitions and treatment of IFD
Proven, probable and possible IFDs were defined according to standard EORTC/MSG criteria [3]. Patients were administered prophylactic, empirical, preemptive, and targeted antifungal drugs according to standard criteria [4]. Serologic biomarkers for IFD including galactomannan and β-d-glucan were not performed. Radiologic imaging including computed tomography (CT) scan was performed as clinically indicated, particularly in patients with abnormalities on chest radiography. However, routine surveillance CT scans were not performed.
Results
Patients
During the study period, 534 patients received echinocandins (caspofungin, N = 55; micafungin, N = 306; anidulafungin, N = 173). Of these, four patients (0.7 %) developed proven IFDs, and four patients (0.7 %) developed possible IFDs while receiving echinocandins.
Proven IFD
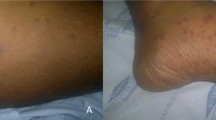
Four cases of proven IFDs were observed (patients 1–4, Table 1). In two cases (patients 2 and 3), the echinocandins were used prophylactically, in view of high-risk features including refractory or relapsed leukemia, antecedent HSCT, and prolonged neutropenia. In two cases (patients 1 and 4), echinocandins were administered empirically for neutropenic fever not responding to broad-spectrum antibiotics. The incriminated organisms were C. parapsilosis in two cases, C. parapsilosis and C. glabrata in one case (patient 1, Table 1 and Fig. 1a, b), and Fusarium species in one case (patient 2, Table 1 and Fig. 1c–e). Patients received a median of 39 (30–69) days of echinocandin before IFD was diagnosed. It is important to note that each of these patients had received the purine analog clofarabine in combination with high-dose cytarabine immediately before the breakthrough IFD [5]. Liposomal amphotericin B was used as salvage antifungal treatment, together with posaconazole (patient 1) and voriconazole (patient 2). Two patients did not improve and died. The remaining two patients responded, but died of refractory leukemia and bacterial sepsis.
Breakthrough invasive fungal disease during echinocandin therapy. a Patient 1, showing a necrotic lesion over the right little toe (white arrow) and septic thrombophleblitis (white arrow). b Patient 1, amputated toe specimen showing the presence of fungal elements in the thrombus with invasion into the vessel wall (arrow) (Grocott stain). c Patient 2, with computed tomographic scan showing a left apical pulmonary mass (arrow) with central cavitations, which was surrounded by ground-glass consolidation (halo sign). d Patient 2, with painful necrotic lesions on the shin (arrows). e Patient 2, with necrotic shin lesion showing multiple branching and septated fungal hyphae, which with the clinical picture was consistent with infection with Fusarium species (Grocott stain). f Patient 5, showing a large right lung mass with halo sign (arrow). g Patient 7, showing multiple cavitary lesions (arrows). h Patient 8, with computed tomographic scan showing multiple hepatic microabscesses (arrows) and multiple splenic lesions, which in the given clinical context were suggestive of hepatosplenic candidiasis
Possible IFD
Four cases of possible IFDs were identified (patients 5–8, Table 1). All cases had lesions localized to the chest. In two cases (patients 5 and 6), the echinocandin was used empirically for neutropenic fever not responding to broad-spectrum antibiotics (Fig. 1f, patient 5). In two cases, the echinocandins were administered prophylactically, in view of previous allogeneic HSCT and high-dose corticosteroid treatment for ongoing graft-versus-host disease (GVHD) (Fig. 1g, patient 7). It is noteworthy that in these two cases, the absolute neutrophil count was normal. In one of the cases, the radiologic features were suggestive of chronic disseminated candidiasis (hepatosplenic candidiasis) (Fig. 1h, patient 8). With treatment, three patients responded with radiologic improvement. However, two patients finally died of complications of GVHD. One patient never became afebrile and died a month later. Notably, two of the four patients had also received clofarabine and high-dose cytarabine immediately before IFD.
Discussion
This is to date one of the largest series of patients treated with echinocandins, where breakthrough IFDs were studied. Because echinocandins are active against Candida and Aspergillus species, the two most common organisms causing IFD, breakthrough fungal infections during their usage are unusual. However, zygomycetes, Cryptococcus neoformans, Fusarium species, and Trichosporon species are innately resistant to echinocandins [1], so that breakthrough IFD caused by these fungi might be expected. Surprisingly, only very few cases of breakthrough IFD due to these organisms have been described. In a review of breakthrough IFD during echinocandins therapy [2], where 37 isolates were recovered, only 13 (35 %) were due to Trichosporon species (N = 8), zygomycetes (N = 3), C. neoformans (N = 2), and Fusarium species (N = 1). As information was pooled from 20 studies [2], the data might also be confounded by the prevalent spectra of IFD in these geographic areas.
In this study, we observed a low frequency of breakthrough IFD (8/534, 1.5 %). Candida was involved in three of the four proven cases. It might also be implicated in at least one of the four possible cases, where the radiologic features were suggestive of hepatosplenic candidiasis. C. parapsilosis was identified in all three proven cases. In fact, C. parapsilosis has been reported to be the most common non-albicans Candida causing blood stream infections [6]. We had not performed antifungal susceptibility tests. However, C. parapsilosis is known to be less sensitive to echinocandins [7]. Furthermore, C. parapsilosis tends to have higher minimal inhibitory concentrations to anidulafungin and micafungin than other Candida species [7]. Recent studies have also noted a small but detectable increase in the proportion of Candida species that have decreased echinocandin susceptibility [7].
Echinocandins target the Fks1p subunit of β-1,3-d-glucan synthase, which is encoded by three related genes, FKS1, FKS2, and FKS3 [8]. It has been proposed that a naturally occurring proline-to-alanine substitution at amino acid position 660 of FKS may be responsible for the reduced echinocandin susceptibility of C. parapsilosis [9]. The finding of the breakthrough C. parapsilosis infection in our patients might therefore be related to its inherent reduced echinocandin susceptibility. On the other hand, mutations of the FKS at certain hotspots may also render the Fks1p subunit resistant to echinocandins [8]. The presence of FKS mutations has in fact been defined occasionally in breakthrough Candida infection during echinocandin therapy.
Four cases were possible IFDs. Because we had not measured fungal biomarkers (galactomannan and β-d-glucan), the diagnoses could not be upgraded to probable IFD. In all cases, the chest appeared to be the site of the infection. In one case, additional features were suggestive of hepatosplenic candidiasis. In the other cases, aspergillus might be involved. Acquired resistance of Aspergillus species to echinocandins due to FSK gene mutation appears to be rare [8]. However, breakthrough invasive mold infections during caspofungin therapy caused predominantly by Aspergillus species have been reported, with a frequency as high as 7.3 % [10]. Because fungal cultures were not available for the majority of cases in that study, the sensitivity of the Aspergillus isolates to caspofungin could not be fully evaluated. Proposed mechanisms of breakthrough IFD in these instances included inadequate serum levels of caspofungin and severe immunosuppression. In our cohort, caspofungin had been used sparingly. Even with the presumption that the three possible cases of IFD were all due to mold infections, the frequency would still be very low at <1 % in our cohort, when micafungin and anidulafungin were the predominant echinocandins used.
An important observation in this study is that, of the eight cases of IFD, six patients had received clofarabine and high-dose cytarabine, making this regimen the most significant risk factor. Clofarabine is a purine analog targeting both lymphoid and myeloid cells [5]. Treatment with clofarabine therefore leads to profound neutropenia and lymphopenia. Although neutropenia can be alleviated with the use of hematopoietic growth factors, lymphopenia may be very prolonged, which may explain the predisposition to IFD in the present series. Our experience therefore indicates that after clofarabine treatment, an echinocandin may not be adequate for antifungal coverage, particularly for the less susceptible Candida species. An azole that covers both yeast and mold, such as posaconazole, may be a better option.
The three echinocandins were not used at the same rate. Micafungin was used most frequently, followed by anidulafungin and caspofungin. Because this was not a prospective study, drugs were used according to the discretion of the physician in charge. In general, micafungin was started in most cases, with anidulafungin prescribed for patients with impaired hepatic function [1]. Caspofungin was least prescribed, because of concerns of its drug interactions [1], particularly with ciclosporin in our HSCT patients. In other words, patients receiving caspofungin tended to be on fewer medications, implying that they were less complicated. Hence, the fact that we were seeing more breakthrough IFD in patients treated with anidulafungin and micafungin might be related to the preferential usage of these echinocandins in patients with more complicated clinical courses, particularly because of hepatic GVHD and drug-induced liver dysfunction, where anidulafungin and to a slightly lesser extent micafungin could have a better safety profile. Hence, it is unlikely that our data reflected a differential tendency of the three echinocandins in leading to breakthrough IFD.
The results in this study were confounded by some problems. Serologic biomarkers for IFD, including galactomannan and β-d-glucan, were not assayed because of resource constraints. The availability of these tests would have upgraded our cases from possible to probable IFD. This might also have somewhat affected the frequency of breakthrough infections. For neutropenic fever, we have adopted an empirical approach [11] so that surveillance CT scans were not performed. However, CT scans were performed when indicated, even if chest radiographs only showed subtle changes. Finally, postmortem examinations had not been performed, and this might have led to a very small number of IFD undiagnosed.
To conclude, breakthrough IFDs were uncommon during echinocandin treatment and appeared to be caused predominantly by less sensitive Candida species. Use of the purine analog clofarabine is an important risk factor.
References
Chen SC, Slavin MA, Sorrell TC (2011) Echinocandin antifungal drugs in fungal infections: a comparison. Drugs 71:11–41
Sun HY, Singh N (2010) Characterisation of breakthrough invasive mycoses in echinocandin recipients: an evidence-based review. Int J Antimicrob Agents 35:211–8
De Pauw B, Walsh TJ, Donnelly JP et al (2008) European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–21
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ et al (2011) Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis 52:e56–93
Tse E, Leung AY, Sim J et al (2011) Clofarabine and high-dose cytosine arabinoside in the treatment of refractory or relapsed acute myeloid leukaemia. Ann Hematol 90:1277–81
Ortega M, Marco F, Soriano A et al (2011) Candida species bloodstream infection: epidemiology and outcome in a single institution from 1991 to 2008. J Hosp Infect 77:157–61
Pfaller M, Boyken L, Hollis R et al (2011) Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Candida species to anidulafungin, caspofungin, and micafungin. J Clin Microbiol 49:624–9
Pfaller MA (2012) Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125(1 Suppl):S3–13
Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS (2008) A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother 52:2305–12
Pang KA, Godet C, Fekkar A et al (2012) Breakthrough invasive mould infections in patients treated with caspofungin. J Infect 64:424–9
Chan TS, Hwang YY, Gill H et al (2013) Antifungal drug usage in haematologic patients during a 4-year period in an Asian university teaching hospital. Intern Med J 43(5):541–6
Conflict of interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, T.S.Y., Gill, H., Hwang, YY. et al. Breakthrough invasive fungal diseases during echinocandin treatment in high-risk hospitalized hematologic patients. Ann Hematol 93, 493–498 (2014). https://doi.org/10.1007/s00277-013-1882-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1882-2