Abstract
Patients with primary refractory or relapsed acute myeloid leukemia (AML) have a dismal prognosis. We report a retrospective single center analysis of aplasia-inducing chemotherapy using fludarabine, cytarabine, and amsacrine (FLAMSA) followed by reduced-intensity conditioning (RIC) for allogeneic hematopoietic cell transplantation (HCT) in 62 consecutive primary refractory or relapsed AML patients. Two-year event-free survival and overall survival (OS) were 26 and 39 %, respectively. Risk stratification according to cytogenetic and molecular genetic markers showed superior survival in patients in the intermediate-1 risk group (2-year OS 70 %) compared to the intermediate-2 risk (2-year OS 34 %, p = 0.03) and adverse risk (2-year OS 38 %, p = 0.06) group. The use of HLA-matched versus HLA-mismatched donors had no significant influence on survival (p = 0.98). Two-year OS in the elderly subgroup defined by age ≥60 years was 31 % compared to 46 % in the group of younger patients <60 years (p = 0.19). Cumulative incidence of non-relapse mortality at 2 years adjusted for relapse as competing risk was 20 % for patients <60 years and 26 % for older patients (p = 0.55). Chronic graft-versus-host disease was associated with a statistically significant superior survival (p < 0.01). FLAMSA-RIC followed by allogeneic HCT enables long-term disease-free survival in primary refractory or relapsed AML even in the elderly patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary refractory or relapsed acute myeloid leukemia (AML) is associated with a dismal prognosis. Approximately, one third of patients younger than 60 years and 50 % of older patients with newly diagnosed AML fail to achieve complete remission (CR) with standard induction chemotherapy [1]. Among patients achieving CR, around half of patients relapse depending on their risk stratification (age, cytogenetics, molecular markers, etc.) [2]. Currently, several salvage chemotherapeutic regimens are under investigation in the context of clinical trials but sustained CR is virtually never achieved. To date, allogeneic hematopoietic cell transplantation (HCT) is the only potentially curative approach. Conventional myeloablative conditioning (MAC) regimens could not overcome the poor prognosis due to high non-relapse mortality (NRM) and high incidence of relapse. Recently, promising results in patients with treatment refractory or relapsed AML have been reported using a sequential treatment approach with aplasia-inducing chemotherapy consisting of fludarabine, cytarabine, and amsacrine (FLAMSA) followed after 3 days of rest by reduced-intensity conditioning (RIC) for allogeneic HCT. Schmid and colleagues proposed RIC with 4-Gy total body irradiation (TBI) and cyclophosphamide (CY) and reported a 2-year overall survival (OS) of 40 % and leukemia-free survival of 37 % [3]. A French group presented similar results using TBI/CY or busulfan (BU)/CY for RIC after aplasia-inducing chemotherapy with FLAMSA [4]. This sequential treatment strategy with effective reduction of the leukemic burden followed by RIC-HCT to confer a potent graft-versus-leukemia (GVL) effect allows new perspectives even for an elderly patient population with poor performance status. We present our single center experience with this approach for the treatment of primary refractory and relapsed AML.
Materials and methods
We report a retrospective analysis of our single center experience with FLAMSA-RIC in primary refractory or relapsed AML. We searched our institutional database for patients receiving FLAMSA-RIC within the last 7 years, and details on characteristics and clinical course were confirmed by retrospective chart review. Informed consent was obtained from all patients before being included in the study. This study is in accordance with the ethical standards of the institutional review board and with the Helsinki Declaration of 1975, as revised in 2008.
Patient characteristics
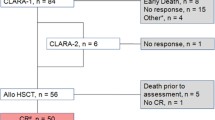
Patient characteristics are summarized in Table 1. We identified and analyzed 62 consecutive patients (28 females, 34 males) with a median age of 55 years (range 20–72) transplanted after FLAMSA-RIC in our institution from 2005 to 2012. Primary refractory AML was defined as blast persistence in bone marrow aspirates on day 14 after first or second induction treatment. Relapsed AML was defined as >5 % blasts in the bone marrow aspirate in patients who achieved a cytological CR after first or second induction treatment. Data on molecular and cytogenetic markers were available in 46 and 55 patients, respectively. Patients were classified to different risk groups based on the recommendation by Doehner and others [5].
HLA typing and donors
Patients and their donors were tested for HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 by high-resolution molecular typing methods. As donor, 10/10 HLA-matched related (MRD) and unrelated donors (MUD) or 1–2 antigen/allele mismatched related (MMRD) or unrelated donors (MMUD) were used. All grafts consisted of G-CSF mobilized peripheral blood stem cells (PBSC).
Conditioning regimens, transplantation, and GVHD prophylaxis
FLAMSA (fludarabine 30 mg/m2, cytarabine 2,000 mg/m2, and amsacrine 100 mg/m2 on days −12 to −9) was uniformly used as aplasia-inducing salvage therapy followed after 3 days of rest by RIC consisting of either FLU/BU (fludarabine 30 mg/m2 on days −5 to −4, busulfan 0.8 mg/kg twice on day −6, and 0.8 mg/kg four times daily on days −5 to −4, n = 12), TBI/CY (4 Gy on day −5, cyclophosphamide 60 mg/kg on days −4 to −3, n = 31), or BU/CY (busulfan 0.8 mg/kg once on day −6, 0.8 mg/kg four times daily on day −5, and 0.8 mg/kg three times on day −4, cyclophosphamide 60 mg/kg for mismatched and unrelated donors or 40 mg/kg for MRD on days −3 to −2, n = 19). On day 0, all patients received fresh or cryopreserved PBSC. HCT was performed after a median of 75 (range 38–197, primary refractory AML) and 329 (range 63–2,100, relapsed AML) days following induction treatment. Cyclosporine A (2005–2007, n = 8, plasma level 200–250 ng/ml, start day −1) or tacrolimus (2007–2012, n = 54, 10–15 ng/ml, start day −1) combined with mycophenolate mofetil (MMF, 1 g twice daily, start day +1) and anti-thymocyte globuline (ATG-Fresenius®, 10 mg/kg for related donors and 20 mg/kg for unrelated donors, days −3 to −1) were used as graft-versus-host disease (GVHD) prophylaxis. MMF was tapered 500 mg every week from day +30 on. Calcineurin inhibitors were tapered from day +60 to day +90 if no acute GVHD occurred. Acute GVHD was graded according to the Glucksberg criteria [6]. Chronic GVHD was graded according to the revised Seattle classification [7].
Monitoring of patients for engraftment, chimerism, infections, and donor lymphocyte infusions
Hematopoietic donor cell chimerism in mononuclear cells was monitored in all patients using microsatellite markers as described previously [8]. Engraftment and immune reconstitution were assessed by peripheral blood counts and flow cytometry. The reconstitution of CD4+ cells was monitored in 2–4 weeks intervals in the early post-transplant period, later every 3 months. Neutrophil engraftment was defined as the first of two consecutive days with the absolute neutrophil count over 500 neutrophils/μL. Platelet engraftment was defined as the first of two consecutive days with more than 20,000 platelets/μL without platelet transfusion. All patients were treated according to our institutional transplant guidelines for antiviral, antifungal, and antimicrobial prophylaxis.
As reported previously, prophylactic donor lymphocyte infusions (DLI) were given from day +120 in patients who were not receiving immunosuppressant without evidence of GVHD [9]. Otherwise, patients received additional DLI in case of mixed chimerism, relapse, or disease progression. Peripheral blood mononuclear cells were freshly obtained from the donor or cryopreserved and adjusted to a defined CD3+ T lymphocyte content and given after various time points after transplantation.
Statistical analysis
For statistical analysis, GraphPad Prism 4.03 (GraphPad Software Inc., La Jolla, CA, USA), JMP 10.0 (SAS Institute Inc., San Francisco, CA, USA), and R (R Foundation for Statistical Computing, Vienna, Austria) were used. OS and event-free survival (EFS) were calculated using the Kaplan–Meier estimate. OS was defined as time from HCT to death from any cause, and EFS was defined as time from HCT to relapse, progression, or death from any cause, whichever occurred first. If no event occurred, data were censored and the time from HCT until last patient contact was considered. The log-rank test was used for the comparison of Kaplan–Meier estimates between different groups of patients with a significance level α of 0.05. Proportional hazards were calculated with the semiparametric Cox regression model. Cumulative incidence curves for NRM and relapse were adjusted for competing risks [10]. All p values are two sided.
Results
Disease status and risk stratification
At the time of HCT, 36 patients were refractory after first induction chemotherapy and 26 had an untreated relapse after achieving initially cytological CR. Forty-eight patients could be classified with regards to their genetic risk profile to the intermediate-1 (n = 15), intermediate-2 (n = 17), or adverse (n = 16) risk groups. No patient had a favorable risk profile.
Graft content, engraftment, and donor chimerism
All patients received fresh or cryopreserved grafts containing a median of 5.4 × 106 CD34+ cells/kg (range 2.6–18.3 × 106/kg) on day 0. Eleven patients were transplanted from a MRD, 22 from a MUD, 25 from a MMUD, and 4 from a MMRD. Neutrophil and thrombocyte engraftment occurred after a median of 17 days (range 10–77) and 22 days (range 8–154). Due to early relapse or transplant-related death, no neutrophil and thrombocyte engraftment was noted in two and four patients, respectively. Thirty-eight, 65, and 71 % of patients alive had a complete donor chimerism in the peripheral blood on days +20, +60, and +100, respectively.
Infections, toxicity, GVHD, relapse, and non-relapse mortality
Toxicity related to FLAMSA-RIC is summarized in Table 2. Incidence of acute GVHD ≥ II and chronic GVHD (limited n = 12, extensive n = 4) was 21 and 26 %, respectively (Table 3). A total of 32 patients relapsed. Cumulative incidence of relapse at 2 years adjusted for NRM as competing risk was 52 %. Relapse occurred after a median of 103 days (range 23–931) upon HCT. Causes of death were relapse (n = 22), infections (n = 5), multiple organ failure (MOF, n = 6), GVHD (n = 2), cerebral hemorrhage (n = 1), and progressive multifocal leukoencephalopathy (n = 1). Cumulative incidence of NRM at 2 years adjusted for relapse as competing risk was 22 % for all patients, 20 % for patients <60 years, and 26 % for older patients (p = 0.55).
Donor lymphocyte infusions
In 16 patients, DLI (2.0 × 106–1.4 × 108 CD3+ cells/kg) were administered after a median of 167 days (range 72–922). Only three patients received DLI prophylactically as intended: one died from relapse, one died from MOF in CR, and one is alive after a follow-up of 1,310 days. Thirteen patients received DLI for relapse or mixed chimerism of which seven patients relapsed before day +120. Finally, nine patients died from relapse. Four patients are alive with a follow-up after HCT of 244 to 1,133 days.
Outcome, overall, and event-free survival
Current OS is 25/62 patients with a median follow-up of 525 days (range 66–2,329) of patients alive resulting in a Kaplan–Meier estimated 2-year EFS and OS of 26 and 39 % (Fig. 1), respectively. Risk stratification according to cytogenetic and molecular genetic markers showed superior survival in patients in the intermediate-1 risk group (2-year OS 70 %) compared to the intermediate-2 risk group (2-year OS 34 %, p = 0.03), and adverse risk (2-year OS 38 %, p = 0.06) group (Fig. 2a). Two-year OS was similar in patients being refractory to induction chemotherapy compared to those with relapse after first remission (40 versus 36 %, p = 0.44). Two-year OS in de novo AML was 45 % compared to 31 % in secondary AML (p = 0.29). The outcome in the elderly subgroup defined by age ≥60 years (n = 24, median age 67 years) was not significantly different to the group of younger patients (n = 38, median age 46 years) with a 2-year OS of 31 versus 46 %, respectively (p = 0.19, Fig. 2b). Patients with a blast count <20 % in the bone marrow prior to conditioning with FLAMSA-RIC had a similar outcome compared to higher blast counts (2-year OS 50 versus 33 %, p = 0.58). No significant difference between the three RIC regimens (2-year OS 46 % of FLU/BU, 32 % of TBI/CY, 44 % of BU/CY) has been observed (Fig. 2c). Mismatched HCT was not associated with an adverse outcome after 2 years compared to matched HCT (38 versus 39 %, p = 0.98). Tacrolimus-based immunosuppression showed a trend towards an improved 2-year OS (43 versus 13 %, p = 0.08). Cumulative incidence of NRM and relapse at 2 years adjusted for each other as competing risks were not significantly different if grafts from matched or mismatched donors were used (19 versus 26 %, p = 0.60 and 52 versus 51 %, p = 0.58, respectively). CD34+ peripheral blood stem cell count of the graft showed no significant influence on survival (2-year OS 40 % for CD34+ ≤5.4 × 106/kg versus 44 % for CD34+ >5.4 × 106/kg, p = 0.83). Chronic GVHD was associated with a statistically significant improved 2-year OS (68 versus 27 %, p < 0.01, Fig. 2d), whereas outcome for acute GVHD ≥ II was similar compared to acute GVHD < II (2-year OS 37 versus 43 %, p = 0.58). We performed a multivariate analysis taking into account significant factors identified by univariate analysis. Only the intermediate-1 risk group compared to the intermediate-2 risk group was associated with a significantly improved OS (risk ratio 0.26, p = 0.01). The occurrence of chronic GVHD showed a risk ratio of 0.41 (p = 0.05).
a Overall survival according to genetic risk profiles. b Outcome in the elderly subgroup defined by 60 years and older compared to younger patients. c Influence of the reduced-intensity conditioning regimen on survival. d Association of chronic GVHD with improved overall survival. OS overall survival, HCT hematopoietic cell transplantation, Int intermediate, FLU fludarabine, BU busulfan, TBI total body irradiation, CY cyclophosphamide, GVHD graft-versus-host disease, bold p values indicate significant differences between the Kaplan–Meier estimates (p < 0.05)
Discussion
Primary refractory AML and AML relapsed in first CR are both characterized by a dismal prognosis. After first relapse, 5-year OS is 11 % [2, 11]. Allogeneic HCT may provide the highest chance of long-term disease-free survival as GVL effects contribute to the eradication of persistent malignant cells. However, results of salvage allogeneic HCT have been disappointing due to both relapse and high NRM caused by conventional MAC. Duval et al. report a 3-year OS of 19 % in 1,673 patients treated with MAC prior to allogeneic HCT for relapsed or primary refractory AML. A poor Karnofsky score turned out to be one strong adverse pretransplantation variable among others [12]. Two thirds of patients with AML are older than 60 years, and high-risk features (unfavorable cytogenetics, secondary AML, poor performance status) are commonly found in the elderly [13]. Besides, age itself is an independent prognostic parameter and comorbidities limit therapeutic options particularly in MAC [14]. In the era of RIC, lower therapy-related toxicity enables this potentially curative treatment option for the more comorbid and older patient population with a lower performance status [15]. A sequential approach with aplasia-inducing chemotherapy followed by RIC after a short period of rest combines the advantages of effective cytoreduction with the benefits of RIC-HCT. We report our single center experience with aplasia-inducing FLAMSA followed up within 3 days by RIC (FLU/BU, TBI/CY, or BU/CY) for allogeneic HCT.
The 2-year OS of 39 % and 2-year NRM of 22 % found in our cohort confirm the initial report by Schmid et al. with 2-year OS of 40 % and 2-year NRM of 22 % using FLAMSA followed by TBI/CY in refractory AML [3]. It has been shown previously that 2-year OS with RIC only (4 × 2 Gy TBI and 4× fludarabine 30 mg/m2) in AML patients in CR versus untreated or refractory disease is 81 and 21 %, respectively [16]. This underlines the need for prior reduction of the leukemic burden to improve survival. Currently, different HCT protocols and salvage regimens are under investigation. For example, Detrait et al. reported a 2-year OS of 30 % using FLAMSA-TBI/CY or FLAMSA-BU/CY with a significantly lower relapse rate in the FLAMSA-BU/CY cohort [4]. Buchholz et al. investigated a cytoreductive regimen with clofarabine and cytarabine followed by RIC (TBI/CY) for relapsed (n = 9) or refractory (n = 6) AML in a total of 27 patients with a 2-year OS of 56 % [17]. The latter study also included low-risk patients which have to be taken into account for any comparisons.
Published experiences of FLAMSA-RIC in patients above the age of 65 years are scarce. In our cohort, 24 patients were ≥60 years and 17 patients were ≥65 years old. Two-year OS and 2-year NRM were similar in patients ≥60 years compared to younger patients. This indicates that FLAMSA-RIC is also a realistic treatment option for older adults with an acceptable NRM.
In our study, 27 patients were treated for primary refractory or relapsed secondary AML (evolved from myelodysplastic syndromes or therapy related). Both are more frequent in the elderly and are associated with adverse cytogenetics and a poor prognosis [1, 18, 19]. Although not statistically significant, we noted an inferior outcome in patients with secondary AML compared to de novo AML which is consistent with the more aggressive biology of secondary AML [20]. In addition, high-risk molecular and cytogenetic markers were associated with a significantly reduced 2-year OS. In our study, HLA matching did not influence survival, whereas Cannas et al. and Detrait et al. reported an improved outcome with better HLA match in their French retrospective multicenter studies [4, 21]. A more potent GVL effect might be counterbalanced by a higher incidence of acute GVHD.
As reported previously, GVHD is associated with a lower incidence of relapse and improved survival after allogeneic HCT [22–24]. We could demonstrate that a subgroup of our patients with chronic GVHD had a statistically significant improved 2-year OS of 68 %. As shown in Fig. 2d, a plateau is reached after 14 months. This might indicate long-lasting disease-free survival. Interestingly, survival was similar in patients with acute GVHD ≥ II compared to acute GVHD < II. Fatal course of acute GVHD might contribute to NRM and thus outbalance the beneficial GVL effect [25]. Cyclosporine A-based immunosuppression showed a trend towards a reduced survival. Our data demand cautious interpretation as only the first eight patients of this analysis were treated with a cyclosporine A from 2005 to 2007. All patients treated later received tacrolimus. Improved supportive care and increasing experience with this preparative regimen within the following years might be important confounders.
Early discontinuation of immunosuppression and administration of DLI are two options to promote GVL effects but have to be weighed against the higher risk of GVHD. We used DLI in 16 patients, but ten finally died from relapse. With relapse or acute GVHD occurring before day +120, only three patients received DLI prophylactically as intended. A recent study suggests that DLI are of value for consolidation in patients in CR after HCT, whereas delayed application results in a poor outcome [26]. For this reason, DLI should be administered routinely after HCT in the absence of GVHD in a high-risk AML patient. With a minimal number of potentially persistent malignant leukemic cells, alloreactivity of the donor immune system might be sufficient to hamper conversion of minimal residual disease into frank relapse and consequently translate into improved survival [27]. In a study of Schmid and colleagues, 17 patients qualified for prophylactic DLI which were repeatedly given from day +120 after HCT in the absence of GVHD, and finally, a 3-year OS of 87 % could be achieved [3]. With a relatively high percentage of patients relapsing before day +120, we propose a routine bone marrow aspirate on day +30 particularly in patients with a molecular marker. In case of minimal residual disease or mixed chimerism, early discontinuation of immunosuppression and administration of DLI could be scheduled. Improved survival has been observed by Dominietto and colleagues in patients treated with DLI as soon as minimal residual disease occurred [28].
The relatively low number of patients and the retrospective character of this single center study limit the validity of our results. A prospective, multicenter, randomized controlled trial to compare FLAMSA-RIC regimens with conventional MAC in patients with refractory or relapsed AML is certainly overdue. However, preexisting comorbidities, age, pretreatment intensity, and ethical considerations limit randomization possibilities.
In conclusion, aplasia-inducing chemotherapy with FLAMSA followed by RIC for primary refractory or relapsed AML enables long-term disease-free survival regardless of age. Nevertheless, more effort has to be done in order to improve the dismal prognosis of these high-risk AML patients.
References
Lowenberg B, Downing JR, Burnett A (1999) Acute myeloid leukemia. N Engl J Med 341:1051–1062
Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF et al (2005) Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 23:1969–1978
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D et al (2006) Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 108:1092–1099
Detrait MY, Chevallier P, Sobh M, Guillaume T, Thomas X, Morissetet S et al (2011) Outcome of high-risk and refractory AML/MDS patients receiving a FLAMSA sequential chemotherapy regimen followed by reduced-intensity conditioning (RIC) and allogeneic hematopoeitic stem cell transplantation (allo-HSCT). Blood (ASH Annual Meeting Abstracts) 118: abstract 1957
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK et al (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115:453–474
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18:295–304
Lee SJ, Vogelsang G, Flowers ME (2003) Chronic graft-versus-host disease. Biol Blood Marrow Transplant 9:215–233
Bader P, Beck J, Frey A, Schlegel PG, Hebarth H, Handgretinger R et al (1998) Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant 21:487–495
Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ (2005) Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol 23:5675–5687
Scrucca L, Santucci A, Aversa F (2007) Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 40:381–387
Rowe JM, Li X, Cassileth PA, Appelbaum FR, Schiffer CA, Wiernik PH et al (2005) Very poor survival of patients with AML who relapse after achieving a first complete remission: The Eastern Cooperative Oncology Group experience. Blood (ASH Annual Meeting Abstracts) 106: abstract 546
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM et al (2010) Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 28:3730–3738
Estey EH (2012) Acute myeloid leukemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 87:89–99
Schoch C, Kern W, Schnittger S, Buchner T, Hiddemann W, Haferlach T (2004) The influence of age on prognosis of de novo acute myeloid leukemia differs according to cytogenetic subgroups. Haematologica 89:1082–1090
Federmann B, Faul C, Vogel W, Kanz L, Bethge WA (2010) Outcome of patients aged ≥60 after allogeneic hematopoietec cell transplantation: age has no impact on survival. Blood (ASH Annual Meeting Abstracts) 116: abstract 3540
Stelljes M, Bornhauser M, Kroger M, Beyer J, Sauerland MC, Heinecke A et al (2005) Conditioning with 8-Gy total body irradiation and fludarabine for allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Blood 106:3314–3321
Buchholz S, Dammann E, Stadler M, Krauter J, Beutel G, Trummer A et al (2012) Cytoreductive treatment with clofarabine/ara-C combined with reduced-intensity conditioning and allogeneic stem cell transplantation in patients with high-risk, relapsed, or refractory acute myeloid leukemia and advanced myelodysplastic syndrome. Eur J Haematol 88:52–60
Kayser S, Dohner K, Krauter J, Kohne CH, Horst HA, Held G et al (2011) The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2,853 adult patients with newly diagnosed AML. Blood 117:2137–2145
Klepin HD, Balducci L (2009) Acute myelogenous leukemia in older adults. Oncologist 14:222–232
de Witte T, Oosterveld M, Span B, Muus P, Schattenberg A (2002) Stem cell transplantation for leukemias following myelodysplastic syndromes or secondary to cytotoxic therapy. Rev Clin Exp Hematol 6:72–85
Cannas G, Huynh A, Morisset S, Sobh M, Raus N, Garban F et al (2009) Safety and efficacy of FLAMSA regimen followed by allogeneic hematopoietic stem cell transplantation from related and unrelated donors in high risk acute leukemia and MDS patients: a study from the Societe Française De Greffe De Moelle Et De Therapie Cellulaire (SFGM-TC) Registry. Blood (ASH Annual Meeting Abstracts) 114: abstract 4335
Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED (1981) Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med 304:1529–1533
Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L et al (1989) Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood 73:1720–1728
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al (1990) Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75:555–562
Ringden O, Hermans J, Labopin M, Apperley J, Gorin NC, Gratwohl A (1996) The highest leukaemia-free survival after allogeneic bone marrow transplantation is seen in patients with grade I acute graft-versus-host disease. Acute and Chronic Leukaemia Working Parties of the European Group for Blood and Marrow Transplantation (EBMT). Leuk Lymphoma 24:71–79
Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R et al (2012) Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood 119:1599–1606
Craddock CF (2008) Full-intensity and reduced-intensity allogeneic stem cell transplantation in AML. Bone Marrow Transplant 41:415–442
Dominietto A, Pozzi S, Miglino M, Albarracin F, Piaggio G, Bertolotti F et al (2007) Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood 109:5063–5064
Acknowledgments
The authors would like to thank Diana Kilian for maintaining our HCT database. We would also like to thank Susanne Scheible, Stem Cell Lab, University Children’s Hospital, Tuebingen for providing data about donor lymphocyte infusions.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
DS and BF contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Schneidawind, D., Federmann, B., Faul, C. et al. Allogeneic hematopoietic cell transplantation with reduced-intensity conditioning following FLAMSA for primary refractory or relapsed acute myeloid leukemia. Ann Hematol 92, 1389–1395 (2013). https://doi.org/10.1007/s00277-013-1774-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1774-5