Abstract
Diffuse large B cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin lymphoma and accounts for approximately 30 % of newly diagnosed lymphoid neoplasms in Western countries, and 40–50 % in China. A better understanding of the biology of DLBCL is needed for the development of potential therapeutic agents that target specific intracellular pathways. In this study, expression of the important components of the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway and their clinical significance were investigated in 73 DLBCL cases. The effect of rituximab alone or combined with the PI3K/AKT/mTOR pathway inhibitor rapamycin was further evaluated in the DLBCL cell lines. A total of 73 patients were identified, including 45 men and 28 women aged 18 to 78 years (median age 50 years). Of these patients, p-AKT was positive in 40 cases (54.8 %), p-p70S6K in 34 cases (46.6 %), and p-4E-BP1 in 33 cases (45.2 %). Activation of the PI3K/AKT/mTOR pathway was related to poor disease outcome in DLBCL patients treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) but not in those treated with rituximab-CHOP. Rituximab combined with rapamycin synergically downregulated the PI3K/AKT/mTOR signaling pathway. Western blot analysis revealed a baseline activation status of the PI3K/AKT/mTOR pathway in DLBCL cell lines, with high levels of p-AKT, p-mTOR, in addition to downstream molecules p-p70S6K and p-4E-BP1. The results indicate that the PI3K/AKT/mTOR pathway is a potentially important signaling route and an unfavorable prognostic factor for DLBCL. Patients with PI3K/AKT/mTOR activation experience a more rapidly deteriorating clinical course with poor treatment response and decreased survival time. Addition of rituximab could downregulate PI3K/AKT/mTOR activation, reversing its negative effect on chemotherapy-treated patients. In addition, our results indicate that the combination of rituximab and inhibition of the activated PI3K/AKT/mTOR pathway could be a promising target for DLBCL therapeutic intervention in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Diffuse large B cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin lymphoma and accounts for approximately 30 % of newly diagnosed lymphoid neoplasms in Western countries [1] and 40–50 % in China [2]. Although disease outcome has been significantly improved by combining the monoclonal anti-CD20 antibody rituximab with anthracycline-based cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen, response to treatment is heterogeneous and prognosis is often unpredictable [3]. In addition to the clinical prognostic model, the International Prognostic Index (IPI), molecular profiling based on DNA microarray dividing DLBCL into germinal center (GC)-type and non-GC subtypes has proven to be an efficient predictor of prognosis [4, 5]. Given the genetic heterogeneity of DLBCL, research focusing on the dysregulation of cell signaling pathways may provide further insights into lymphoma cell biology and identify novel therapeutic targets.
Recent studies have highlighted the aberrant expression of the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway and its role in controlling tumor cell proliferation and survival in lymphoid malignancies, including DLBCL [6, 7]. AKT is a serine/threonine protein kinase that mediates various downstream effects of PI3-kinase. It plays a central role in signaling by the PI3K pathway, by regulating many biological processes, such as proliferation, cell growth, and apoptosis [8]. As an important downstream target of AKT, mTOR plays a critical role in cell cycle progression by phosphorylating the ribosomal protein S6 kinase (p70S6K) and the eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), which are the two major proteins involved in the regulation of protein synthesis [9, 10]
Although agents targeting these molecules and pathways have progressed from preclinical models to early clinical trials, little is known about what might predict the response and outcome to these agents in DLBCL patients treated with immunochemotherapy. In this study, the expression of the important components of the PI3K/AKT/mTOR signaling pathway and their clinical significance were investigated in 73 DLBCL cases. The effect of rituximab alone or combined with the PI3K/AKT/mTOR pathway inhibitor rapamycin was further evaluated in the DLBCL cell line SU-DHL-4 and DB. The results showed that PI3K/AKT/mTOR signaling pathway activation in DLBCL may be considered a prognostic biomarker and a promising target for therapeutic intervention.
Methods
Patients
From January 2001 to June 2007, 73 newly diagnosed, adult patients with de novo DLBCL, who received CHOP or rituximab (R)-CHOP therapy with curative intent at the Shanghai Institute of Hematology, were included in this retrospective study. Histological diagnoses and disease classification were performed according to the World Health Organization classification [1]. Informed consent was obtained from all patients in accordance with the regulations of the Shanghai Jiao Tong University School of Medicine Institutional Review Boards.
Cells and reagents
The DLBCL cell lines SU-DHL-4 (CRL-2957) and DB (CRL-2289) were obtained from the American Type Culture Collection (Bethesda, MD). The DLBCL cell line Ly-10 was a gift from Dr Feng R (Department of Hematology, Nanfang Hospital affiliated to Southern Medical University, Guangzhou, China). Cells were maintained in RPMI-1640 medium supplemented with 10 % heat-inactivated fetal bovine serum in a humidified atmosphere of 95 % air and 5 % CO2 at 37 °C.
Rituximab (10 mg/mL) was obtained from Roche (Grenzach, Germany). Rapamycin was purchased from Cell Signaling Technology (Beverly, MA) and dissolved in DMSO as a stock solution of 100 μM.
Immunohistochemistry
Immunohistochemical analyses were performed on 3-μm-thick formalin-fixed, paraffin-embedded sections using antibodies against phospho-AKTThr308 (p-AKTThr308; Cell Signaling Technology; 1:50), phospho-p70S6KThr389 (p-p70S6KThr389; Cell Signaling Technology; 1:50), and phospho-4E-BP1Thr37/46 (p-4E-BP1Thr37/46; Cell Signaling Technology; 1:60) with the Dako Envision Detection System (Dako, Glostrup, Denmark). All antibodies required antigen retrieval in citrate buffer (10 mM, pH 6.0). Positive cells were counted in ten randomly selected high-power fields with a ×40 objective. p-AKT, p-p70S6K, and p-4E-BP1 showed identifiable positive tumor cells with moderate (golden brown) or strong (brown) intensity. Other cells stained very weakly (grayish), and these cells were considered negative. In every staining set, negative controls were applied by omission of the primary antibody. Reactive lymphoid hyperplasia tissue served as positive control. DLBCL subtypes were identified by immunohistochemical analysis. Immunohistochemical procedures were based on those previously described by Hans et al. [11]. Paraffin sections of tumor samples were stained with antibodies against CD10 (Novocastra, Newcastle, UK; 1:80), BCL-6 (Dako, Glostrup, Denmark; 1:10), and MUM1/IRF4 (Dako; 1:40). All sections were independently reviewed by two pathologists.
Western blot
Cells were lysed in 200 μL lysis buffer (0.5 M Tris–HCl, pH 6.8, 2 mM EDTA, 10 % glycerol, 2 % SDS, and 5 % β-mercaptoethanol). Protein extracts (20 μg) were electrophoresed on 10 % SDS polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with 5 % nonfat dried milk in Tris-buffered saline and incubated for 2 h at room temperature with the appropriate primary antibody, followed by a horseradish peroxidase-conjugated secondary antibody. The immunocomplexes were visualized using a chemiluminescence phototope-horseradish peroxidase kit. Antibodies against AKT, p-AKTThr308, mTOR, phospho-mTORSer2448 (p-mTORSer2448), p70S6K, p-p70S6KThr389, 4E-BP1, and p-4E-BP1Thr37/46 were obtained from Cell Signaling Technology. Detection of B-actin (Sigma, St. Louis, MO) was performed to ensure equivalent protein loading.
Response to treatment
Treatment response was evaluated according to the International Workshop criteria [12], based on physical examination, relevant laboratory tests, computer tomography scans, and bone marrow biopsy results. Compared with previous results obtained at diagnosis, tumor response was classified as either complete response (CR), unconfirmed complete response (CRu), partial response (PR), stable disease, or progressive disease. The overall response (OR) rate was calculated as CR or CRu, plus PR. Outcome was expressed in terms of progression-free survival (PFS) and overall survival (OS). PFS was calculated as the time from the date of initial diagnosis to the date of progression, relapse, death, or last follow-up. OS was calculated from the date of diagnosis until death due to any cause or last follow-up.
Statistical analysis
Patient characteristics and complete remission rates were compared by the chi-square and Fisher’s exact tests. The nonparametric Spearman r correlation coefficient was used to test correlations between different variables. Survival functions were estimated using the Kaplan–Meier method and compared using the logrank test. All P values were based on two-sided tests and values <0.05 were considered significant. All statistical analyses were performed using SPSS statistical software (SPSS Inc, Chicago, IL).
Results
The PI3K/AKT/mTOR pathway was activated in DLBCL
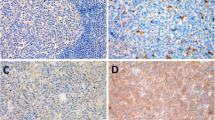
A total of 73 patients were identified, including 45 men and 28 women aged 18 to 78 years (median age 50 years). Of these patients, p-AKT was positive in 40 cases (54.8 %), p-p70S6K in 34 cases (46.6 %), and p-4E-BP1 in 33 cases (45.2 %) (Fig. 1). Immunostaining for p-AKT was cytoplasmic, whereas with p-p70S6K and p-4E-BP1, both cytoplasmic and perinuclear staining were observed. Regarding the correlation between these biomarkers, p-AKT was significantly associated with the expression of p-p70S6K and p-4E-BP1 as a continuous variable (r > 0.5, P < 0.001).
Immunohistochemistry study of PI3K/AKT/mTOR pathway on paraffin tissue sections of malignant lymph nodes from patients with DLBCL (original magnification ×40). a H&E stain presenting diffuse large neoplastic cells. b Negative control. c p-AKT with diffuse cytoplasmic and membrane staining of neoplastic cells. d p-p70S6K with both cytoplasmic and perinuclear accentuation of neoplastic cells. e p-4E-BP1 with diffuse cytoplasmic and membrane staining of neoplastic cells
The immunophenotypic subtypes were defined according to the results obtained from immunohistochemical analysis for the expression of CD10, BCL-6, and MUM1/IRF4. Accordingly, a total of 41.1 % of the patient cohort was classified as GC and 58.9 % as non-GC.
The major clinical and laboratory findings of the patients grouped according to p-AKT expression status are summarized in Table 1. The presence of p-AKT was significantly associated with more frequent presentation in patients with advanced stages (III–IV) of the disease (P = 0.023). Although there was a higher proportion of patients with p-AKT expression in the high-risk group than in the low-risk group, this was not statistically significant (P = 0.225). There was no association between the expression of p-AKT and age, sex, performance status, lactate dehydrogenase (LDH) level, number of extranodal sites, or immunophenotypic subtype (P > 0.05). Expression of p-P70S6K and p-4E-BP1 did not show any significant correlation with any of the clinical variables analyzed.
P-AKT expression was related to poor disease outcome in DLBCL patients treated by CHOP but not in those treated by R-CHOP
All the patients received a median of six cycles (range, four to eight cycles; the majority (95.4 %) received six cycles) of either CHOP (30/73) or R-CHOP (43/73) therapy without dose adjustments at 21-day intervals. After completion of initial therapy, patients were routinely observed every 3 months for the first 2 years, and every 6 months thereafter. Patients who experienced disease recurrence or progression during treatment subsequently received salvage treatment regimens. At the time of analysis, 32 (43.8 %) patients were dead, and 41 (56.2 %) patients were alive at last contact. The overall median follow-up period for the surviving patients was 30 months (range, 10–66 months), 27 months (10–64 months) for the CHOP group, and 32 months (13–66 months) for the R-CHOP group. The OR rate was 74.0 % (54/73) for the entire population. Treatment response was superior in the R-CHOP group (83.7 %, 36/43), compared to that in the CHOP group (60.0 %, 18/30; P = 0.031).
We examined the association between clinical outcome and expression of p-AKT, and its expression was negatively correlated with the likelihood of achieving remission. The overall response rate was 60.0 % (24/40) for the p-AKT-positive cohort of patients, compared to 91.0 % (30/33) for those who were p-AKT negative (P = 0.003). Univariate analysis showed that p-AKT expression was significant in predicting both inferior PFS (3-year estimate, 47.8 vs. 75.6 %; P = 0.006) and OS (3-year estimate, 54.5 vs. 84.6 %; P = 0.005) (Fig. 2).
The data were further analyzed according to the different treatment regimens: p-AKT expression was significantly associated with a poorer response in the CHOP group (37.5 vs. 85.7 %; P = 0.011), but not in the R-CHOP group (75.0 vs. 94.7 %; P = 0.112). In the CHOP group, there were significant differences between p-AKT-positive patients and p-AKT-negative patients for PFS (37.6 vs. 74.0 %; P = 0.008) and OS (45.3 vs. 76.5 %; P = 0.011). However, in the R-CHOP group, the difference was not statistically significant, both for PFS (53.2 vs.74.0 %; P = 0.165) and OS (60.6 vs. 85.8 %; P = 0.152) (Fig. 3), but there was a tendency for an inferior outcome for the p-AKT-positive cohort of patients (Fig. 3c, d)
The expression of p-P70S6K and p-4E-BP1 did not have statistical significance in treatment response and survival (P > 0.05). However, there was a tendency for an inferior outcome for the p-4E-BP1-positive patients.
Activation of the PI3K/AKT/mTOR pathway was observed in a DLBCL cell line and downregulated after treatment with rituximab alone or with a combination of rituximab and the mTOR inhibitor rapamycin
To evaluate the effects of rituximab on the PI3K/AKT/mTOR signaling pathway, the expression and phosphorylation of PI3K/AKT/mTOR downstream targets were evaluated in SU-DHL-4, DB, and Ly-10 cells by Western blot analysis after 48 h. Analysis revealed a baseline activation status of the PI3K/AKT/mTOR pathway in the three DLBLC cells, with high levels of p-AKT and p-mTOR, in addition to downstream molecules p-p70S6K and p-4E-BP1. Treatment with rituximab (20 μg/mL) led to an obvious reduction of p-AKT level in all the three cell lines. The p-mTOR level was reduced in SU-DHL-4 and Ly-10 while minimal effected in DB. The p-p70S6 level was reduced in DB and Ly-10 while minimal effected in SU-DHL-4. The p-4E-BP1 level was reduced in SU-DHL-4 while minimal effected in DB and Ly-10. So, we hypothesized that combining rituximab with the mTOR inhibitor rapamycin may enhance the inhibiting effect of the PI3K/AKT/mTOR pathway. Cells were then treated with rituximab (20 μg/mL) and rapamycin (25 nM) for 48 h. Co-exposure caused significant downregulation of p-AKT and p-mTOR, and consequently phosphorylation of p70S6K and 4E-BP1 was also decreased. There were no changes observed in the total levels of AKT, mTOR, p70S6K, and 4E-BP1 (Fig. 4).
Effect of rituximab combined with rapamycin in SU-DHL-4, DB, and Ly-10 cell lines. Cells (1 × 107/mL) were treated with rituximab (20 μg/mL) ± rapamycin (25 nM) for 48 h and incubated at 37 °C, and total cell lysates were prepared as described in “Methods”. The cell lysates were examined by Western blotting for various unphosphorylated and phosphorylated proteins of the pathway. β-actin was used as control for loading
Discussion
Despite improvements in the OS of DLBCL patients with the routine addition of rituximab therapy, one third of the patients have disease that is either refractory to initial therapy or relapses after standard therapy [13]. The PI3K/AKT/mTOR signaling pathway has been demonstrated to be constitutively activated in several lymphomas including DLBCL [6, 7]. The serine–threonine kinase AKT plays a central role in the PI3K/AKT/mTOR pathway. Activation of AKT (p-AKT) mediates a range of pro-survival signals for anti-apoptosis, proliferation, and cell growth via mTOR, which signals to its downstream effectors S6 kinase/ribosomal protein S6 and 4EBP-1/eIF-4E to control protein translation [14]. The phosphorylated AKT has been found to be abnormally activated in the majority of primary human lymphomas and hematopoietic cell lines [15]. Uddin et al. evaluated the immunohistochemical expression of p-AKT using tissue microarray and correlated it with survival. Their univariate analysis (no data regarding therapy) found that p-AKT-positive patients had inferior survival (P = 0.05), but not after adjustment for IPI [6]. In this retrospective, immunohistochemical study on 73 DLBCL patients, we showed p-AKT expression in lymphoma tissue and a strong association between its presence and its downstream targets p-p70S6 and p-4E-BP1, further confirming that the PI3K/AKT/mTOR pathway is activated in DLBCL. The p-AKT-positive patients had a higher clinical stage (P = 0.023), but we did not find any relationship between the expression of p-AKT and age, sex, performance status, LDH level, number of extranodal sites, or immunophenotypic subtype.
To determine whether p-AKT is related to DLBCL disease progression, we compared the expression of p-AKT with treatment response and survival. Our data revealed that expression of p-AKT was significantly correlated with a decreased response rate (P = 0.003), along with a shorter PFS (P = 0.006) and OS (P = 0.005), according with the Uddin’s finding [6]. Interestingly, the expression of p-AKT was significantly related to disease outcome only in patients treated with CHOP, but not in those treated with R-CHOP. While the difference for R-CHOP was not statistically significant, there was a tendency for an inferior outcome after R-CHOP (Fig. 3c, d). Such an inferior outcome after adjustment for clinical prognostic factors (sex and IPI) for patients with a high p-AKT expression has recently been demonstrated by Hasselblom et al. [16]. Noteworthily, in their study on 106 DLBCL patients, the immunochemotherapy included R-CHOP-21 and R-CHO(E)P-14, with or without consolidating radiotherapy/ASCT according to age-adjusted IPI. Moreover, high p-AKT expression did not significantly predict worse survival when the median value or the 25th percentile was used as cutoff point. As the prognostic role of p-AKT could be of great clinical interest in the era of immunochemotherapy, our finding needs to be confirmed in future study using larger cohorts of patients and, ideally, in a perspective randomized clinical trial. Rituximab mediates its antitumor activity via multiple mechanisms that include complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and induction of apoptosis after CD20 cross-linking [17]. Our study found that rituximab treatment downregulated the expression of p-AKT, in agreement with the study on B-NHL cell lines by Suzuki et al. [18], where treatment with rituximab inhibited the PI3K/AKT signaling pathway, resulting in inhibition of Bcl-xL expression, another important anti-apoptotic member of Bcl-2 family. It has been demonstrated that inhibition of the AKT pathway by rituximab results in the sensitization of B-NHL cells to drug-induced apoptosis [17]. This may explain how the negative prognostic effect of p-AKT expression could be overcome when the patient received chemotherapy combined with rituximab.
Ansell et al. recently demonstrated that the pretreatment expression of p4EBP1 was significantly associated with shorter time to progression in patients with relapsed or refractory mantle cell lymphoma (MCL) receiving temsirolimus (a drug similar to rapamycin) and rituximab [19]. In our experiments, univariate analysis showed that p-4E-BP1 expression was not significant in predicting both PFS and OS (P > 0.05). Key differences between their results and ours were patients (relapsed or refractory MCL vs. de novo DLBCL), treatment strategy, sample size, and follow-up time. However, we found there was a tendency for an inferior outcome for the p-4E-BP1-positive cohort of patients.
Although the initial response rate to rituximab is high, relapse is frequently observed, and close to 60 % of previously rituximab-responding patients do not benefit from re-treatment. Rituximab resistance represents a significant barrier to immunotherapy for DLBCL, and the underlying mechanism is not well understood. Several approaches to overcome this resistance are under evaluation, including combining rituximab with other drugs that interfere with the activated signaling pathways. Rapamycin is a highly specific inhibitor of mTOR and is associated with decreased phosphorylation of its downstream targets p-p70S6K and p-4E-BP1 [20]. It has been suggested that rapamycin blocks cells at the G1 phase via the inhibition of activation of p70S6K and 4E-BP1 [21]. We further analyzed the effect of rituximab combined with rapamycin in three DLBCL cell lines and found significant decrease in p-AKT and p-mTOR, and inhibition of p-p70S6K and p-4E-BP1. Therefore, coadministration of rituximab with rapamycin results in an enhanced effect in inhibiting p-AKT, p-mTOR, and downstream molecules, interrupting the PI3K/AKT/mTOR signaling pathway and might be an attractive treatment option for DLBCL.
In conclusion, our results indicate that the PI3K/AKT/mTOR pathway is a potentially important signaling route and an unfavorable prognostic factor for DLBCL. Patients with PI3K/AKT/mTOR activation experience a more rapidly deteriorating clinical course with poor treatment response and decreased survival time. Addition of rituximab could downregulate PI3K/AKT/mTOR activation, reversing its negative effect on chemotherapy-treated patients. In addition, our results indicate that the combination of rituximab and inhibition of the activated PI3K/AKT/mTOR pathway could be a promising target for DLBCL therapeutic intervention in the future.
References
Jaffe ES, Harris NL, Stein H, Vardiman JW (2001) World Health Organization classification of tumors: pathology and genetics of tumors of hematopoetic and lymphoid tissues. IARC, Lyon
Li JM, Wang L, Shen Y et al (2007) Rituximab in combination with CHOP chemotherapy for the treatment of diffuse large B cell lymphoma in Chinese patients. Ann Hematol 86:639–645
Mey U, Hitz F, Lohri A, Pederiva S, Taverna C, Tzankov A, Meier O, Yeow K, Renner C (2012) Diagnosis and treatment of diffuse large B-cell lymphoma. Swiss Med Wkly 142:0. doi:10.4414/smw.2012.13511
Alizadeh AA, Eisen MB, Davis RE et al (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503–511
Rosenwald A, Wright G, Chan WC et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 20:1937–1947
Uddin S, Hussain AR, Siraj AK et al (2006) Role of phosphatidylinositol 3′-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood 108:4178–4186
Wanner K, Hipp S, Oelsner M, Ringshausen I, Bogner C, Peschel C, Decker T (2006) Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol 134:475–484
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657
Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA (2003) Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 17:590–603
Morgensztern D (2005) McLeod HL.PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs 16:797–803
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES et al (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275–282
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A, Hagenbeek A et al (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244
Friedberg JW (2011) Relapsed/refractory diffuse large B-cell lymphoma. Hematol Am Soc Hematol Educ Program 2011:498–505
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274
Fillmore GC, Wang Q, Carey MJ, Kim CH, Elenitoba-Johnson KS, Lim MS (2005) Expression of Akt (protein kinase B) and its isoforms in malignant lymphomas. Leuk Lymphoma 46:1765–1773
Hasselblom S, Hansson U, Olsson M et al (2010) High immunohistochemical expression of p-AKT predicts inferior survival in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Br J Haematol 149(4):560–568
Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99:754–758
Suzuki E, Umezawa K, Bonavida B (2007) Rituximab inhibits the constitutively activated PI3K-Akt pathway in B-NHL cell lines: involvement in chemosensitization to drug-induced apoptosis. Oncogene 26:6184–6193
Ansell SM, Tang H, Kurtin PJ et al (2011) Temsirolimus and rituximab in patients with relapsed or refractory mantle cell lymphoma: a phase 2 study. Lancet Oncol 12(4):361–368
Fingar DC, Blenis J (2004) Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23:3151–3171
Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, Sawyers CL, Lichtenstein AK (2004) AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem 279:2737–2746
Acknowledgments
This work was supported in part by the Shanghai Commission of Science and Technology (grants 44107025 and 05DZ19317), the National Natural Science Foundation of China (grants 30570777 and 30750335), the Chinese National High Tech Program (grant 863:2006AA02A301), the Shanghai Commission of Science and Technology (grants 08410708800 and 11140901200), and the Program for Outstanding Young Teachers in Universities of Shanghai (JDY09084).
Conflict of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, ZZ., Xia, ZG., Wang, AH. et al. Activation of the PI3K/AKT/mTOR pathway in diffuse large B cell lymphoma: clinical significance and inhibitory effect of rituximab. Ann Hematol 92, 1351–1358 (2013). https://doi.org/10.1007/s00277-013-1770-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1770-9