Abstract
Acute myeloid leukaemia (AML) is a heterogeneous disease with dismal outcome. Sunitinib is an orally active inhibitor of multiple tyrosine kinase receptors approved for renal cell carcinoma and gastrointestinal stromal tumour that has also been studied for AML in several clinical trials. However, the precise mechanism of sunitinib action against AML remains unclear and requires further investigation. For this purpose, this study was conducted using human AML cell lines (HL60 and KG-1) and AML patients’ mononucleated cells. Sunitinib induced G1 phase arrest associated with decreased cyclin D1, cyclin D3, and cyclin-dependent kinase (Cdk)2 and increased p27Kip1, pRb1, and p130/Rb2 expression and phosphorylated activation of protein kinase C alpha and beta (PKCα/β). Selective PKCα/β inhibitor treatment abolished sunitinib-elicited AML differentiation, suggesting that PKCα/β may underlie sunitinib-induced monocytic differentiation. Furthermore, sunitinib increased pro-apoptotic molecule expression (Bax, Bak, PUMA, Fas, FasL, DR4, and DR5) and decreased anti-apoptotic molecule expression (Bcl-2 and Mcl-1), resulting in caspase-2, caspase-3, caspase-8, and caspase-9 activation and both death receptor and mitochondria-dependent apoptosis. Taken together, these findings provide evidence that sunitinib targets AML cells through both differentiation and apoptosis pathways. More clinical studies are urgently needed to demonstrate its optimal clinical applications in AML.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukaemia (AML) is a heterogeneous disorder. With growing understanding of its pathogenesis, cytotoxic chemotherapy with or without follow-up with hematopoietic cell transplantation is the primary treatment for AML. Although much effort has been made concerning AML treatment, the prognosis has not improved substantially in the past decade and still remains dismal [1].
Among all the subtypes of AML, acute promyelocytic leukaemia (APL) is an exception, because with the introduction of all-trans retinoic acid (ATRA), a well-known cell differentiation inducer, to conventional cytotoxic agents, the outcome of patients with APL is greatly superior those with non-promyelocytic AML [2]. Studies have shown that the complete remission rate can reach 90 % when APL patients receive induction and consolidation therapy with regimens composed of ATRA, arsenic trioxide (ATO), and anthracycline-based chemotherapy [3]. In addition, ATO has emerged as an option for relapse patients because it can resensitise patients to the differentiation effects of ATRA, resulting in secondary remission [4, 5]. Unfortunately, ATRA and ATO are not able to induce non-promyelocytic AML cell differentiation successfully, so the majority of patients with AML do not benefit from these differentiation agents. How to treat non-APL AML successfully with differentiation agents other than ATRA and ATO remains an obstacle and requires further investigation. Several agents have been investigated, such as gefitinib, epigenetic agents, and CCAAT/enhancer binding proteins [6]. Among potential agents, gefitinib is a tyrosine kinase inhibitor targeting epidermal growth factor receptor-tyrosine kinase. The initial positive results from gefitinib suggest that tyrosine kinase inhibitors can potentially induce AML cell differentiation and apoptosis. Sunitinib may be another candidate that harbours this efficacy.
Sunitinib is an orally administered small molecule that inhibits the signal transduction pathways responsible for cell proliferation through blockade of the split-kinase domain family of receptor tyrosine kinases, including vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and the fms-related tyrosine kinase (FLT3) receptor [7]. It is currently approved for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumour refractory to imatinib [8]. Several studies have investigated the potential of sunitinib as an AML treatment. One such study conducted by O'Farrell et al. [9] demonstrates that administration of sunitinib in the MV4:11 orthotopic model of human AML results in sustained inhibition of FLT3 signalling and prolonged patient survival. A phase I study conducted by Fiedler et al. [10] also demonstrates that sunitinib as a monotherapy may induce partial response in refractory AML, providing preliminary evidence for its clinical application in AML.
Although a study conducted by Nishioka et al. [11] has demonstrated that sunitinib can induce AML cell monocytic differentiation independent of FLT3 gene status, the molecular mechanisms for sunitinib-induced AML cell differentiation and apoptosis remain largely unclear. Therefore, we conducted the present study to investigate the effector mechanisms of sunitinib-induced human AML cell growth arrest, differentiation, and apoptosis in two AML cell lines (HL60 and KG-1 cells). Patient-derived primary AML blast cells were also studied to strengthen the clinical association of the findings. Our results indicated that sunitinib may act not only as an effective leukaemic differentiation agent but also an apoptosis inducer in AML cells. The results of our study also established a molecular rationale suggesting that sunitinib may have therapeutic potential in patients with AML.
Materials and methods
Reagents
Sunitinib was provided by Pfizer pharmaceutical company (New York, NY). It was dissolved in dimethyl sulfoxide for in vitro studies. Anti-CD11b, anti-CD11c, anti-CD14, anti-CD18, anti-CD54, anti-Fas, and anti-Fas ligand antibodies were purchased from BD Biosciences PharMingen (San Diego, CA). Anti-phospho-protein kinase C (PKC)α/β, anti-cleaved caspase-3, anti-cleaved caspase-8, anti-cleaved caspase-9, and anti-Mcl-1 were purchased from Cell Signalling Technology (Danvers, MA). Anti-β-actin, anti-Bax, anti-Bak, anti-Bcl-2, anti-phospho-PKCα, anti-phospho-PKCβ, anti-pRb1, anti-p130/Rb2, anti-cyclin D1, anti-cyclin D3, and anti-Cdk2 were purchased from Santa Cruz Biochemicals (Santa Cruz, CA). Anti-DR4, anti-DR5, and anti-p27Kip1 were obtained from Abcam (Cambridge, UK). Go6976 (a selective PKCα/β inhibitor) and anti-p53 upregulated modulator of apoptosis (PUMA) antibody were purchased from Calbiochem (La Jolla, CA). Caspase-2, caspase-3, caspase-8, caspase-9, and caspase-12 activity assay kits were purchased from R&D Systems (Minneapolis, MN). A caspase-2 inhibitor (Z-VDVAD-FMK), caspase-3 inhibitor (Z-DEVD-FMK), caspase-8 inhibitor (Z-IETD-FMK), and caspase-9 inhibitor (Z-LEHD-FMK) were purchased from Kamiya (Thousand Oaks, CA). 1α,25-dihydroxycholecalciferol (vitamin D3) was obtained from Sigma-Aldrich (St. Louis, MO).
Cell culture
Human myeloid leukaemia cell lines HL60 and KG-1 were obtained from the American Type Culture Collection (Rockville, MD) and cultured in RPMI 1640 medium supplemented with 5 % foetal bovine serum, 2 mM glutamine, and antibiotics (100 unit/ml penicillin and 100 μg/ml streptomycin) at 37 °C in a 5 % CO2-humidified atmosphere. The culture medium was changed every 2 days. Viable cells were counted with a haemocytometer using the trypan blue dye exclusion method.
Patients
Primary patient-derived AML blast cells for in vitro studies were obtained from three patients with newly diagnosed AML. Mononuclear cells were separated by Ficoll–Hypaque density-gradient centrifugation. Investigations were carried out with written consent of the patients. The project was approved by the Ethics Committee of Taichung Veterans General Hospital.
Cell surface CD markers detection
To analyse the cell surface expression of CD markers, cells (2 × 105 cells/ml) were treated with various concentrations of sunitinib for the indicated times. After treatment, cells were washed with phosphate-buffered saline (PBS) and incubated with phycoerythrin-conjugated or fluorescein isothiocyanate (FITC)-conjugated anti-human CD11b, CD11c, CD14, CD18, and CD54 monoclonal antibodies. Negative control was obtained by staining of the cells with mouse IgG1 and IgG2a followed by incubation at 4 °C for 20 min. Quantitative immunofluorescence measurements were conducted using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) and analysed with CELLQuest™ software.
Morphologic investigation
The morphology of HL60 and KG-1 cells was determined by the Wright–Giemsa method. Briefly, cells were harvested and cytocentrifuged onto a microscope slide using a Cytospin (Shandon Southern Instrument Inc.), and the cells were then stained with Giemsa staining solution for 20 min, rinsed with deionised water, air-dried, and observed under a light microscope. The percentage of differentiated cells was determined by counting 200 cells in each treatment.
Nitroblue tetrazolium reduction assay
Cells (2 × 105 cells/ml) were treated with sunitinib for 24 h and then harvested via centrifugation and incubated with an equal volume of 1 % nitroblue tetrazolium (NBT; Sigma-Aldrich, St. Louis, MO) dissolved in PBS containing 200 ng/ml freshly diluted TPA (Sigma-Aldrich, St. Louis, MO) at 37 °C for 30 min in darkness. The reaction was stopped by washing twice with PBS. A light microscope was used to identify NBT-positive cells, which contained blue-black nitroblue diformazan deposits. The percentage of positive cells was determined by counting 200 cells in each sample.
Quantification of apoptosis by annexin V-FITC/propidium iodide labelling
Apoptosis was quantified by detecting surface exposure of phosphatidylserine in apoptotic cells using an annexin V-FITC/propidium iodide (PI) apoptosis detection kit (BD Biosciences, Clontech, Heidelberg, Germany). Cells were seeded in 6-well plates in 2 ml of medium and incubated with sunitinib at the indicated dose for 48 h. Both adherent and floating cells were harvested, treated according to the manufacturer’s instructions, and analysed by flow cytometry. The annexin V+/PI− (early stage) and annexin V+/PI+ (late stage) apoptotic cell populations were measured.
Quantification of apoptotic cells by terminal transferase-mediated dUTP-fluorescein nick end labelling assay
Cells were treated with sunitinib for the indicated time periods, washed twice with PBS, fixed in 2 % paraformaldehyde for 20 min, and then permeabilised with 0.1 % Triton X-100/PBS for 30 min at room temperature. After washing with PBS, the terminal transferase-mediated dUTP-fluorescein nick end labelling (TUNEL) assay was performed according to the manufacturer’s instructions (Boehringer Mannheim, Germany). Cells were incubated in the TUNEL reaction buffer in a 37 °C humidified chamber for 1 h in the dark, then rinsed twice with PBS and incubated with DAPI (1 mg/ml) at 37 °C for 10 min. The stained cells were visualised using a fluorescence microscope. The fluorescence intensity change was monitored under a fluorescence microscope or analysed by flow cytometry. TUNEL-positive cells were considered apoptotic.
Cell cycle distribution analysis by flow cytometry
Cells were seeded in 6-well plates and treated with sunitinib for the indicated times. The cells were collected, fixed with cold ethanol, and incubated on ice for 30 min. Cells were then resuspended in PI-containing solution (50 μg/ml PI, 0.1 % Na citrate, and 0.1 % Triton X-100) and placed in the dark at 4 °C overnight. Flow cytometric analysis was performed the following day. Each experiment was performed in triplicate.
Protein preparation and western blot analysis
Cells were cultured with sunitinib for the indicated times. After treatment, both adherent and floating cells were harvested, washed twice with ice-cold PBS, and lysed in ice-cold-modified RIPA buffer. After 30-min incubation on ice, cells were centrifuged at 100,000×g for 30 min at 4 °C, and supernatants were collected. Protein concentration was determined using the Bradford method. For western blot analysis, equal amounts of total protein were loaded onto SDS-polyacrylamide gels, and the proteins were transferred onto a PVDF membrane by electrophoresis (Millipore, Bedford, MA). Immunoblots were analysed using specific primary antibodies. After exposure to a horseradish peroxidase-conjugated secondary antibody for 1 h, proteins were visualised using an enhanced chemiluminescence detection kit (ECL Kits, Amersham Life Science).
Caspase activity assay
Cells lysates obtained from sunitinib-treated (1 μM) or untreated cells were tested for caspase-2, caspase-3, caspase-8, caspase-9, and caspase-12 activity by measuring hydrolysis of a fluorogenic peptide substrate (100 μM) for caspase-2 (VDVAD-AFC), caspase-3 (DEVD-AFC), caspase-8 (IETD-AFC), caspase-9 (LEHD-AFC), and caspase-12 (ATAD-AFC) conjugated to the fluorescent reporter molecule 7-amino-4-trifluoromethyl coumarin (R&D System Minneapolis, MN). Cleavage of the peptide by caspase enzymatic activity releases the fluorochrome (excitation/emission = 405/505 nm). The level of caspase enzymatic activity in the cell lysate was directly proportional to the fluorescence signal detected with a fluorescent microplate reader (Fluoroskan Ascent, Labsystems, Finland).
Statistical analysis
The figures presented in this text are representative of at least three independent experiments with similar results. The results are expressed as the mean ± standard deviation of three independent experiments. Statistical differences were evaluated by Student’s t test and considered significant at the following P values (*P < 0.05; **P < 0.01; ***P < 0.001).
Results
Sunitinib induces G1 cell cycle arrest and apoptosis in leukaemia cells
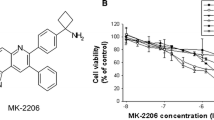
To explore the anti-AML effects of sunitinib, human HL60 and KG-1 cell lines were incubated with various concentrations of sunitinib. Cell viability was determined using the trypan blue exclusion method after 24- and 48-h treatment. As shown in Fig. 1a, sunitinib inhibited HL60 and KG-1 cell growth in a dose- and time-dependent manner with IC50 values of 4.5 and 3.9 μM after 48-h administration, respectively. To investigate the precise action responsible for the sunitinib-mediated antiproliferative effect, the cell cycle distribution profile was examined. As shown in Fig. 1b, exposure of HL60 and KG-1 cells to sunitinib for 24 h led to an accumulation of cells in the G0/G1 phase compared with control cells, coupled with a concomitant decrease in the proportion of cells in the S and G2/M phases. Additionally, a hypodiploid DNA content peak, or sub-G1 population, which is indicative of degraded DNA and a hallmark of apoptosis, was observed following 24 h of high-dose treatment and increased continuously after 48-h incubation (Fig. 1b).
Sunitinib induces G1 cell cycle arrest and apoptosis in leukaemia cells. a Antiproliferative effect of sunitinib. HL60 and KG-1 cells were treated with the indicated concentrations of sunitinib for 24, 48, and 72 h. Cell viability was determined by trypan blue dye exclusion assay. Cells treated with dimethyl sulfoxide were used as control. b Sunitinib causes G1 cell cycle arrest. The proportion of HL60 and KG-1 cells in different cell cycle phases was analysed by flow cytometry after sunitinib treatment for 24 and 48 h. c Induction of apoptosis by sunitinib. HL60 and KG-1 cells were treated with 6 μM sunitinib for 48 h. Apoptosis was examined by flow cytometry with annexin-V and propidium iodide staining. Bars indicate mean, and error bars indicate standard deviation of three independent experiments
Next, co-staining of annexin V and PI was performed. As shown in Fig. 1c, increased annexin V labelling was observed in sunitinib-treated cells, suggesting that HL60 and KG-1 cells underwent apoptosis in response to sunitinib exposure.
Sunitinib regulates G1 phase regulatory molecules
A recent study demonstrated that sunitinib treatment consistently caused cell cycle arrest in the G0/G1 phase and dephosphorylation of the retinoblastoma protein (pRb1) in papillary thyroid cancer cell lines [12]. Therefore, we next analysed the effect of sunitinib on G0/G1-specific regulatory molecules, specifically the G1 cyclins, Cdks, and Cdk inhibitors. As shown in Fig. 2, exposure to sunitinib for 24 h markedly increased the expression of p27Kip1, pRb1, and p130/Rb2 but decreased the expression of cyclin D1, cyclin D3, and Cdk2. However, the expression of Cdk4, Cdk6, cyclin D2, and cyclin E were not affected by sunitinib in either tested AML cell line (data not shown).
Sunitinib-regulated G1 phase regulatory molecules. As a dose-dependent experiment, HL60 and KG-1 cells were treated with different concentrations of sunitinib for 24 h. Cell lysates from untreated or sunitinib-treated HL60 and KG-1 cells was then extracted. G1 phase regulatory proteins p27Kip1, pRb1, p130/Rb2, cyclin D1, cyclin D3, and Cdk2 were analysed by western blot. β-actin was used as a loading control
Sunitinib induces caspase-dependent apoptosis
Because caspase plays a pivotal role in apoptosis [13], we next examined the effect of sunitinib on caspase activity using a fluorogenic substrate assay kit. Sunitinib stimulated the activation of caspase-2, caspase-3, caspase-8, and caspase-9 at 48 h (Fig. 3a). Caspase-12 activity, however, did not change in sunitinib-treated HL60 and KG-1 cells. To further confirm activation of caspase-3, caspase-8, and caspase-9, specific antibodies were used to detect the proteolytically activated fragments of each caspase (Fig. 3b). As expected, the active fragments of caspase-3, caspase-8, and caspase-9 were observed after sunitinib treatment for 48 h. Moreover, pretreatment with inhibitors of caspase-2, caspase-3, caspase-8, and caspase-9 significantly prevented sunitinib-induced apoptosis as demonstrated by a reduction in the sub-G1 population (Fig. 3c), which suggested that both the mitochondria- and death receptor-induced caspase activation pathways were involved in sunitinib-induced apoptosis.
Sunitinib induces caspase-dependent apoptosis. a Sunitinib induced caspase activation. Cell lysates were obtained from HL60 and KG-1 cells treated with 6 μM sunitinib for 48 h to examine caspase-2, caspase-3, caspase-8, caspase-9, and caspase-12 activities by fluorometric caspase assay kits, and b the cleaved caspase-3, caspase-8, and caspase-9 were determined by western blot analysis. c Caspase inhibitors prevented sunitinib-mediated apoptosis. HL60 and KG-1 cells in (a) were pretreated with caspase inhibitors. Apoptotic cells were determined by TUNEL assay. Data are presented as mean ± standard deviation of three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001)
Sunitinib regulates apoptosis-related molecules
Because Bcl-2 family proteins are key factors in controlling the mitochondria-dependent apoptotic pathway [14], we further examined the expression levels of anti- and pro-apoptotic molecules in sunitinib-treated and untreated AML cells. As shown in Fig. 4a, decreased Bcl-2 and Mcl-1 (anti-apoptotic proteins) and increased Bax, Bak, and PUMA (pro-apoptotic proteins) were observed in sunitinib-treated AML cells compared with controls; however, the expression of Bcl-xL did not change after sunitinib treatment (data not shown).
Sunitinib regulates apoptosis-related molecules. a Bcl-2 family proteins. After treatment with increasing concentrations of sunitinib for 48 h, cell lysates were extracted from HL60 and KG-1 cells. Expression of Bcl-2 family proteins Bcl-2, Mcl-1, Bax, Bak, and PUMA were analysed by western blot. b Death receptor superfamily proteins Fas, Fas-L, DR4, and DR5 were also analysed by western blot in the cell lysates extracted in (a). β-actin was used as the loading control
Next, the regulation of death receptor superfamily proteins by sunitinib in AML cells was investigated. As shown in Fig. 4b, Fas, Fas-L, DR4, and DR5 expression significantly increased after sunitinib treatment. In contrast, tumour necrosis receptors I and II were not affected by sunitinib (data not shown). These results indicated that both the Bcl-2 family and the death receptor superfamily proteins were regulated during sunitinib-induced apoptosis in HL60 and KG-1 cells.
Sunitinib induces monocytic differentiation in AML cells
Sunitinib has been shown to induce monocytic differentiation of HL60 and MOLM13 human AML cells [11]. Therefore, the potential of sunitinib to induce cell differentiation in HL60 and KG-1 cells was investigated. Data from flow cytometric analyses demonstrated that the cell surface levels of differentiation markers CD14 and CD11b dose-dependently increased in sunitinib-treated cells compared with controls (Fig. 5a). Next, the cell surface markers of HL60 and KG-1 cells, including CD11b, CD11c, CD14, CD18, and CD54 were examined after 1 μM sunitinib treatment; vitamin D3 treatment was used as a positive control. As shown in Fig. 5b, the proportion of cells expressing CD11b, CD11c, CD14, CD18, and CD54 increased after sunitinib and vitamin D3 treatments compared with untreated group.
Sunitinib induces monocytic differentiation in AML cells. a HL60 and KG-1 cells were treated with various concentrations of sunitinib (0, 0.1, 0.3, and 1 μM) for 24 h. Expression of CD14 and CD11b was analysed by flow cytometry. b Expression of cell surface markers. Cells were treated with 1 μM sunitinib or 30 nM vitamin D3 (Vit D3) for 24 h. The expressions of surface markers were examined by flow cytometry. Untreated cells are shown in white histogram. Sunitinib-treated cells are shown in gray histogram. Vit D3-treated cells are shown in black histogram. c Morphological analysis. Monocytic differentiation of HL60 and KG-1 cells treated with various concentrations of sunitinib for 24 h were investigated with Wright–Giemsa staining. Cells with cytoplasm enlargement, nuclear deformation, and the presence of large bilobate nuclei were defined as demonstrating monocytic differentiation. d Quantification of differentiated cells after Wright–Giemsa staining. Two hundred cells in (c) were counted to quantify differentiated cells. e Monocytic differentiation identified by nitroblue tetrazolium (NBT) reduction. Cells treated in (c) were analysed by NBT reduction assay. Cells demonstrating monocytic differentiation were identified by their intracellular blue formazan deposits. f Two hundred cells in (e) were counted to quantify differentiated cells. Cells treated with dimethyl sulfoxide were used as negative control. Bars indicate mean, and error bars indicate standard deviation of three independent experiments (***P < 0.001)
Furthermore, sunitinib-induced AML cell differentiation was characterised by Wright–Giemsa staining. As shown in Fig. 5c, control HL60 and KG-1 cells were predominantly myeloblasts with round and regular cell margins and large nuclei. However, sunitinib-treated HL60 and KG-1 cells contained bilobate nuclei, decreased cell size, and increased cytoplasm-to-nuclear ratio, which is morphologically consistent with monocytic differentiation. Approximately 76.5 ± 6.3 % of HL60 cells and 87.2 ± 7.5 % of KG-1 cells in the 1 μM sunitinib-treated group displayed evidence of monocytic differentiation, which was significantly higher than those treated with vehicle control (Fig. 5d). NBT assay data further confirmed that sunitinib induced HL60 and KG-1 monocytic differentiation. As shown in Fig. 5d, an increase of cytoplasmic black granules, indicating myeloid cell differentiation, was observed in both HL60 and KG-1 cells after 24-h sunitinib treatment (Fig. 5e). In each experiment, 200 cells were counted three times. On average, approximately 48.5 ± 5.6 % of HL60 cells and 57.3 ± 5.9 % of KG-1 cells were positive for NBT staining following 1 μM sunitinib treatment, which was significantly higher than that in the control group (Fig. 5f).
Association of sunitinib-induced AML cell differentiation with PKC
A number of intracellular signal transduction proteins, including mitogen-activated protein kinase, Janus family kinases, signal transducer and activator of transcription, phosphatidylinositol-3-kinase, Smads, and PKC, regulate the process of myeloid differentiation [15]. To gain insights into the signalling molecules involved in sunitinib-induced differentiation, we applied pharmacological inhibitors of different signalling pathways to examine their effect on differentiation. The results demonstrated that the selective PKCα/β inhibitor Go6976 partially but significantly reduced the sunitinib-stimulated surface expression of CD14 and CD11b (Fig. 6a) in HL60 and KG-1 cells, suggesting that inhibition of PKC could block sunitinib-induced cell differentiation. Results from western blot analysis revealed that phosphorylated PKCα and PKCβ increased in a dose-dependent manner after 2-h sunitinib treatment (Fig. 6b). By contrast, the expression of total PKCα, PKCβ, PKCδ, and phosphorylated PKCδ were not affected by sunitinib (data not shown). These results indicated that sunitinib-induced differentiation of AML cells may be associated with the PKCα/β signalling pathway.
Sunitinib-induced AML cell differentiation is PKC related. a The PKCα inhibitor Go6976 reversed sunitinib-induced cell differentiation. HL60 and KG-1 cells were pretreated with 0.1 μM Go6976 for 2 h, and then 1 μM sunitinib was added for another 24 h. After treatment, expression of CD11b and CD14 was measured using flow cytometry. b Regulation of PKCα/β by sunitinib. AML cells were treated with various concentrations of sunitinib for 2 h. The levels of phosphorylated PKCα and PKCβ proteins were analysed by western blot. β-actin was used as the loading control
Sunitinib induces monocytic differentiation and cell death in patient-derived AML blast cells
To evaluate the potential value of sunitinib as a therapeutic agent in AML, the effects of sunitinib on the differentiation and viability of freshly isolated patient-derived AML blasts were examined. Monocytic differentiation was induced by sunitinib treatment (0.3 and 1 μM) in two (identified as AML FAB-M2) of three patients’ AML blasts, as indicated by the induction of the monocytic differentiation markers CD14 and CD11b (Fig. 7a). The levels of CD14 and CD11b differentiation antigens were unchanged in the blasts isolated from an AML FAB-M5 patient, indicating that sunitinib did not induce differentiation in this sample. Moreover, sunitinib exhibited a strongly cytotoxic effect on the blasts from all three tested patients. Almost all AML blasts died after treatment with 6 μM sunitinib for 48 h. At 3 μM sunitinib treatment, AML blasts decreased to approximately 25 ± 3, 35 ± 6, and 20 ± 5 % of the number in untreated cultures from patients 1, 2, and 3, respectively (Fig. 7b).
Sunitinib induces monocytic differentiation of patient-derived AML blast cells. Mononucleated cells were freshly isolated from three AML patients and then incubated with different concentrations of sunitinib for 24 h. a Expressions of cell surface markers CD14 and CD11b were measured using flow cytometry. Untreated control group is shown in white histogram; sunitinib-treated group is shown in gray histogram. b AML blast cells were treated with 3 and 6 μM sunitinib for 48 h. Cell viability was determined by trypan blue dye exclusion method. White bar indicates untreated control; gray bar indicates cells after 3 μM sunitinib treatment; and black bar indicates cells after 6 μM sunitinib treatment (***P < 0.001)
Discussion
The “two-hit” hypothesis for leukaemogenesis suggests that leukaemia is the result of two classes of mutations. The class I mutation impairs hematopoietic differentiation, and the class II mutation confers a proliferative and/or survival advantage on hematopoietic progenitors [16]. Therefore, induction of differentiation and apoptosis is a potential strategy for AML treatment. In this study, we report that sunitinib inhibited proliferation of human AML cell lines HL60 and KG1 with IC50 values of 4.5 and 3.9 μM, respectively, after 48-h treatment. Additionally, sunitinib induced cell cycle G0/G1 phase arrest, monocytic differentiation, and apoptosis in HL60 and KG-1 cells. These sunitinib-mediated actions were independent of p53 status, because the p53-null HL60 cells [17] exhibited a response to sunitinib treatment comparable to that of KG-1 cells, which have a functional p53. Furthermore, sunitinib also induced monocytic differentiation and cytotoxicity in primary patient-derived AML blasts.
In mammalian cells, a subset of the G1 to S phase transition proteins, D-type cyclins, Rb family proteins, and Cdk inhibitors (including INK4 and Cip/Kip family proteins), trigger this cell cycle progression. D-type cyclins activate their Cdk-4/6 catalytic partners in mid- to late-G1 phase, while cyclin E appears later in G1 and activates its Cdk-2 kinase subunit near the G1/S checkpoint [18]. Furthermore, the mammalian Cdk inhibitor p27Kip1 functionally links to G0/G1-S progression control, while overexpression of p27Kip1 induces cell cycle arrest in the G0/G1 phase [19]. The retinoblastoma (Rb) protein family, including pRb1, p130/Rb2, and p107, are additional negative regulators responsible for controlling G0/G1 phase in the cell cycle by their expression pattern, timing of association with E2Fs, and the cell cycle arrest elicited by their overexpression [20]. A recent report conducted by Yang et al. [21] demonstrated that sunitinib induced cell growth arrest in medulloblastoma via downregulation of cyclin E, cyclin D2, and cyclin D3 and upregulation of p21Cip1. In our study, we found that sunitinib induced G0/G1 phase arrest in AML cells, which may be associated with downregulation of cyclin D1, cyclin D3, and Cdk2 proteins and upregulation of p27Kip1, pRb1, and p130/Rb2. These results suggest that the sunitinib-mediated cell cycle perturbation in AML cells may be the result of a multifaceted attack on multiple target molecules that are critically involved in G0/G1 phase arrest in the cell cycle.
Downregulation of cyclin D1 proteins and upregulation of p27Kip1, pRb1, and p130/Rb2 may also play an important role in monocytic differentiation of AML cells after sunitinib treatment. Studies have shown that Rb family proteins and Cdk inhibitors such as p21Cip1 and p27kip1 can also induce embryonic, neural, or hematopoietic stem cell differentiation [18]. Additionally, mice lacking p27Kip1 demonstrate differentiation defects [22]. In the hematopoietic cell system, accumulation of p27Kip1 also plays a crucial role in erythroid differentiation coupled with G1 arrest [23, 24]. Moreover, upregulation of pRb1 and Rb2/p130 is specifically observed during differentiation in many cellular systems, such as muscle cells [25], adipocytes [26], myeloid cells and regenerating liver [27], osteoblasts [28], keratinocytes [29], astrocytomas and normal astrocytes [30], murine neuroblastoma cells [31], and human neuronal cells [32]. In the present study, we found that p27Kip1, pRb1, and p130/Rb2 expression increased during sunitinib-induced monocytic differentiation; however, further studies are needed to address the exact role of p27Kip1, pRb1, and p130/Rb2 in sunitinib-induced monocyte differentiation. Taking these findings together, we hypothesise that decreased expression of cyclin D1 and increased expression of p27Kip1, pRb1, and p130/Rb2 are not only responsible for G1 phase arrest but also monocytic differentiation in AML cells treated by sunitinib.
This study also revealed that PKCα/β may be important molecules involved in sunitinib-induced monocytic differentiation of AML cells. In the hematopoietic system, PKC activation during monocytic differentiation has been shown [33]. PKCα and PKCβ have also been shown to play an important role in TPA-induced differentiation in U937 cells [34]. Overexpression of PKC-α or PKC-δ promotes TPA-induced macrophage differentiation of 32Dcl3 cells [35]. Additionally, overexpression of PKCα leads to monocytic differentiation in U937 cells [36]. Moreover, G-CSF-induced HL60 cell differentiation is blocked by PKC inhibitors [37]. Our results demonstrated that sunitinib enhanced phosphorylated PKCα and PKCδ activities (Fig. 6b) and sunitinib-induced monocytic differentiation could be partially blocked by the PKCα/δ inhibitor Go6976 (Fig. 6a), suggesting that PKCα/δ may play an important role in sunitinib-induced monocytic differentiation. However, these results cannot exclude the possibility that other molecules are involved in the sunitinib-induced monocytic differentiation of AML cells. A study conducted by Nishioka et al. [11] demonstrated that sunitinib-induced differentiation was accompanied by upregulation of C/EBP-α and downregulation of c-Myc in MOLM13 and HL60 cells. However, in our study, sunitinib did not affect the expression of either C/EBP-α or c-Myc in KG-1 and HL60 cells (data not shown).
The mechanisms underlying sunitinib-induced AML cell apoptosis were also investigated in this study. Sunitinib has been reported to inhibit cell proliferation and induce apoptosis in various cancer cell lines including transformed Ba/F3 cells expressing FLT3 K663Q [38], renal cell carcinoma cells [39], FLT3-mutated MV4-11 and MOLM13 leukaemia cells [40], and human bladder T24 cancer cells [41]. However, the precise mechanisms of sunitinib-induced apoptotic death in AML cells have not been extensively explored. In the current study, sunitinib-induced apoptosis in human AML cells was confirmed by an increased proportion of AML cells in sub-G1 phase and translocation of phosphatidylserine to the cell surface. Many molecules participate in cell apoptosis regulation, among which caspases are the central effectors promoting apoptosis in response to apoptosis-inducing signals from both intrinsic (mitochondrial disruption and endoplasmic reticulum stress) and extrinsic (cell surface death receptor) pathways. Caspase activation during sunitinib-induced apoptosis has been reported in several cancer systems including cervical cancer cells [42], medulloblastoma [21], human urothelial carcinoma TCC-SUP and 5637 cell lines [43], 4 T1 breast cancer cells [44], gastrointestinal stromal T1 cells [45], and hormone refractory prostate cancer [46]. In our study, caspase-2, caspase-3, caspase-8, and caspase-9, were activated by sunitinib-induced apoptosis in AML cells. Treatment with caspase inhibitors conferred partial protection against this effect, suggesting that both mitochondrial and death receptor-ligand signalling pathways may be involved in sunitinib-induced AML cell apoptosis.
Bcl-2 family proteins have been well documented as playing a crucial role in mitochondrial apoptotic pathway regulation. Cell response to apoptotic stimuli critically depends on the balance between pro- and anti-apoptotic Bcl-2 family proteins. Anti-apoptotic proteins in the Bcl-2 family include Bcl-2, Bcl-xL, and Mcl-1, which prevent cell death. On the contrary, pro-apoptotic molecules in Bcl-2 family proteins include Bax, Bak, PUMA, and Bad [14]. Recently, a study conducted by Yoon et al. [47] demonstrated that sunitinib induced cell apoptosis in human bladder cancer HTB5 cells by increasing Bax and Bad pro-apoptotic protein expression. In addition, Bcl-2 downregulation is also observed during sunitinib-induced apoptosis in T24 cells [41]. Ding et al. [48] also demonstrate that sunitinib-induced cell death in both colon cancer SW620 cells and lung cancer 95-D cells is associated with decreased Mcl-1 expression.
In the present study, Bcl-2 and Mcl-1 expression decreased while Bax, Bak, and PUMA increased in sunitinib-induced apoptotic AML cells. These results indicated that mitochondria-triggered apoptosis in sunitinib-treated AML cells may be attributable to downregulation of anti-apoptotic Bcl-2 family members and upregulation of pro-apoptotic Bcl-2 family members. We also found that sunitinib activated caspase-8 and increased Fas, FasL, DR4, and DR5 protein expression. These results provided the first evidence that extrinsic apoptotic pathway (death receptor/caspase-8 system) activation could be an essential mechanism responsible for sunitinib-induced apoptosis in AML cells. However, how these anti- and pro-apoptotic molecules are regulated in sunitinib-treated AML cells remains unclear. The precise mechanisms underlying sunitinib-induced apoptosis still require further investigation.
To characterise the potential clinical application of sunitinib in AML, we examined its cytotoxic and differentiation capacities in patient-derived myeloid blast cells. The results in myeloid blast cells from patients 1 and 2 were basically consistent with those in AML cell lines, suggesting that sunitinib could possibly benefit patients with non-APL AML. However, monocytic differentiation did not significantly increase in myeloid blast cells from patient 3 after sunitinib treatment. One possible explanation for this inconsistent finding is that since patient 3 was diagnosed with acute monocytic leukaemia, sunitinib could not further differentiate the monocytic blast cells. However, more patient-derived myeloid blast cells are still needed to confirm this speculation.
In conclusion, our study provided evidence that the multitarget tyrosine kinase receptor inhibitor sunitinib induced G0/G1 phase arrest, monocytic differentiation, and apoptosis in human AML cell lines HL60 and KG-1. Increased p27Kip1, pRb1, and p130/Rb2 expression and decreased cyclin D1, cyclin D3, and Cdk2 expression may be responsible for both G0/G1 phase arrest in the cell cycle and monocytic differentiation. PKCα and PKCβ may also play a crucial role on sunitinib-induced monocytic differentiation. Moreover, caspase cascade activation (caspase-2, caspase-3, caspase-8, and caspase-9), pro-apoptotic molecule (Bax, Bak, PUMA, DR4, and FasL) upregulation, and anti-apoptotic molecule (Bcl-2 and Mcl-1) downregulation demonstrated that sunitinib-induced apoptosis in AML cells occurred via both intrinsic and extrinsic apoptotic pathways. Well-designed clinical trials are urgently needed to determine the most optimal clinical setting for sunitinib use in patients with non-APL AML.
References
Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS, Koduru PR, Moore JO, Stone RM, Mayer RJ, Feldman EJ, Davey FR, Schiffer CA, Larson RA, Bloomfield CD (2002) Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 100(13):4325–4336. doi:10.1182/blood-2002-03-0772
Petrie K, Zelent A, Waxman S (2009) Differentiation therapy of acute myeloid leukemia: past, present and future. Curr Opin Hematol 16(2):84–91. doi:10.1097/MOH.0b013e3283257aee
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Buchner T, Dohner H, Burnett AK, Lo-Coco F (2009) Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 113(9):1875–1891. doi:10.1182/blood-2008-04-150250
Estey E, Garcia-Manero G, Ferrajoli A, Faderl S, Verstovsek S, Jones D, Kantarjian H (2006) Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 107(9):3469–3473. doi:10.1182/blood-2005-10-4006
Mathews V, George B, Lakshmi KM, Viswabandya A, Bajel A, Balasubramanian P, Shaji RV, Srivastava VM, Srivastava A, Chandy M (2006) Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood 107(7):2627–2632. doi:10.1182/blood-2005-08-3532
Koeffler HP (2010) Is there a role for differentiating therapy in non-APL AML? Best Pract Res Clin Haematol 23(4):503–508. doi:10.1016/j.beha.2010.09.014
Chow LQ, Eckhardt SG (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25(7):884–896. doi:10.1200/JCO.2006.06.3602
Mena AC, Pulido EG, Guillen-Ponce C (2010) Understanding the molecular-based mechanism of action of the tyrosine kinase inhibitor: sunitinib. Anti-Cancer Drugs 21(Suppl 1):S3–S11. doi:10.1097/01.cad.0000361534.44052.c5
O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM (2003) SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 101(9):3597–3605. doi:10.1182/blood-2002-07-2307
Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, Bello CL, Allred R, Manning WC, Cherrington JM, Louie SG, Hong W, Brega NM, Massimini G, Scigalla P, Berdel WE, Hossfeld DK (2005) A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood 105(3):986–993. doi:10.1182/blood-2004-05-1846
Nishioka C, Ikezoe T, Yang J, Yokoyama A (2009) Sunitinib, an orally available receptor tyrosine kinase inhibitor, induces monocytic differentiation of acute myelogenous leukemia cells that is enhanced by 1,25-dihydroxyvitamin D(3). Leukemia 23(11):2171–2173. doi:10.1038/leu.2009.152
Fenton MS, Marion KM, Salem AK, Hogen R, Naeim F, Hershman JM (2010) Sunitinib inhibits MEK/ERK and SAPK/JNK pathways and increases sodium/iodide symporter expression in papillary thyroid cancer. Thyroid 20(9):965–974. doi:10.1089/thy.2010.0008
Ulukaya E, Acilan C, Yilmaz Y (2011) Apoptosis: why and how does it occur in biology? Cell Biochem Funct 29(6):468–480. doi:10.1002/cbf.1774
Kelly PN, Strasser A (2011) The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ 18(9):1414–1424. doi:10.1038/cdd.2011.17
Miranda MB, Johnson DE (2007) Signal transduction pathways that contribute to myeloid differentiation. Leukemia 21(7):1363–1377. doi:10.1038/sj.leu.2404690
Kelly LM, Gilliland DG (2002) Genetics of myeloid leukemias. Annu Rev Genom Hum Genet 3:179–198. doi:10.1146/annurev.genom.3.032802.115046
Konopleva M, Tsao T, Ruvolo P, Stiouf I, Estrov Z, Leysath CE, Zhao S, Harris D, Chang S, Jackson CE, Munsell M, Suh N, Gribble G, Honda T, May WS, Sporn MB, Andreeff M (2002) Novel triterpenoid CDDO-Me is a potent inducer of apoptosis and differentiation in acute myelogenous leukemia. Blood 99(1):326–335
Lange C, Calegari F (2010) Cdks and cyclins link G1 length and differentiation of embryonic, neural and hematopoietic stem cells. Cell Cycle 9(10):1893–1900
Blomen VA, Boonstra J (2007) Cell fate determination during G1 phase progression. Cell Mol Life Sci CMLS 64(23):3084–3104. doi:10.1007/s00018-007-7271-z
Dyson N (1998) The regulation of E2F by pRB-family proteins. Genes Dev 12(15):2245–2262
Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H (2010) Sunitinib induces apoptosis and growth arrest of medulloblastoma tumor cells by inhibiting STAT3 and AKT signaling pathways. Mol Cancer Res MCR 8(1):35–45. doi:10.1158/1541-7786.MCR-09-0220
Tong W, Kiyokawa H, Soos TJ, Park MS, Soares VC, Manova K, Pollard JW, Koff A (1998) The absence of p27Kip1, an inhibitor of G1 cyclin-dependent kinases, uncouples differentiation and growth arrest during the granulosa->luteal transition. Cell Growth Differ 9(9):787–794
Dzikaite V, Kanopka A, Brock JH, Kazlauskas A, Melefors O (2000) A novel endoproteolytic processing activity in mitochondria of erythroid cells and the role in heme synthesis. Blood 96(2):740–746
Tamir A, Petrocelli T, Stetler K, Chu W, Howard J, Croix BS, Slingerland J, Ben-David Y (2000) Stem cell factor inhibits erythroid differentiation by modulating the activity of G1-cyclin-dependent kinase complexes: a role for p27 in erythroid differentiation coupled G1 arrest. Cell Growth Differ 11(5):269–277
Delehouzee S, Yoshikawa T, Sawa C, Sawada J, Ito T, Omori M, Wada T, Yamaguchi Y, Kabe Y, Handa H (2005) GABP, HCF-1 and YY1 are involved in Rb gene expression during myogenesis. Genes Cells 10(7):717–731. doi:10.1111/j.1365-2443.2005.00873.x
Hansen JB, Jorgensen C, Petersen RK, Hallenborg P, De Matteis R, Boye HA, Petrovic N, Enerback S, Nedergaard J, Cinti S, te Riele H, Kristiansen K (2004) Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci U S A 101(12):4112–4117. doi:10.1073/pnas.0301964101
Garriga J, Limon A, Mayol X, Rane SG, Albrecht JH, Reddy EP, Andres V, Grana X (1998) Differential regulation of the retinoblastoma family of proteins during cell proliferation and differentiation. Biochem J 333(Pt 3):645–654
Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang WF, Forrester WC, Hinds PW (2001) The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell 8(2):303–316
Paramio JM, Lain S, Segrelles C, Lane EB, Jorcano JL (1998) Differential expression and functionally co-operative roles for the retinoblastoma family of proteins in epidermal differentiation. Oncogene 17(8):949–957. doi:10.1038/sj.onc.1202031
Galderisi U, Melone MA, Jori FP, Piegari E, Di Bernardo G, Cipollaro M, Cascino A, Peluso G, Claudio PP, Giordano A (2001) pRb2/p130 gene overexpression induces astrocyte differentiation. Mol Cell Neurosci 17(3):415–425. doi:10.1006/mcne.2000.0949
Raschella G, Tanno B, Bonetto F, Negroni A, Claudio PP, Baldi A, Amendola R, Calabretta B, Giordano A, Paggi MG (1998) The RB-related gene Rb2/p130 in neuroblastoma differentiation and in B-myb promoter down-regulation. Cell Death Differ 5(5):401–407. doi:10.1038/sj.cdd.4400359
Chen C, Wells AD (2007) Comparative analysis of E2F family member oncogenic activity. PLoS One 2(9):e912. doi:10.1371/journal.pone.0000912
Meinhardt G, Roth J, Hass R (2000) Activation of protein kinase C relays distinct signaling pathways in the same cell type: differentiation and caspase-mediated apoptosis. Cell Death Differ 7(9):795–803. doi:10.1038/sj.cdd.4400709
Macfarlane DE, Manzel L (1994) Activation of beta-isozyme of protein kinase C (PKC beta) is necessary and sufficient for phorbol ester-induced differentiation of HL-60 promyelocytes. Studies with PKC beta-defective PET mutant. J Biol Chem 269(6):4327–4331
Mischak H, Pierce JH, Goodnight J, Kazanietz MG, Blumberg PM, Mushinski JF (1993) Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-alpha and -delta and not by protein kinase C-beta II, -epsilon, -zeta, and -eta. J Biol Chem 268(27):20110–20115
Noti JD, Reinemann BC, Johnson AK (2001) The leukocyte integrins are regulated by transcriptional and post-transcriptional mechanisms in a leukemic cell that overexpresses protein kinase C-zeta. Int J Oncol 19(6):1311–1318
Deshpande RV, Peterson RH, Moore MA (1997) Granulocyte colony-stimulating factor-induced activation of protein kinase-C in myeloid cells. J Cell Biochem 66(3):286–296
Schittenhelm MM, Yee KW, Tyner JW, McGreevey L, Haley AD, Town A, Griffith DJ, Bainbridge T, Braziel RM, O'Farrell AM, Cherrington JM, Heinrich MC (2006) FLT3 K663Q is a novel AML-associated oncogenic kinase: determination of biochemical properties and sensitivity to Sunitinib (SU11248). Leukemia 20(11):2008–2014. doi:10.1038/sj.leu.2404374
Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H (2009) Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 69(6):2506–2513. doi:10.1158/0008-5472.CAN-08-4323
Nishioka C, Ikezoe T, Yang J, Takeshita A, Taniguchi A, Komatsu N, Togitani K, Koeffler HP, Yokoyama A (2008) Blockade of MEK/ERK signaling enhances sunitinib-induced growth inhibition and apoptosis of leukemia cells possessing activating mutations of the FLT3 gene. Leuk Res 32(6):865–872. doi:10.1016/j.leukres.2007.09.017
Ping SY, Wu CL, Yu DS (2010) Sunitinib can enhance BCG mediated cytotoxicity to transitional cell carcinoma through apoptosis pathway. Urol Oncol. doi:10.1016/j.urolonc.2010.07.001
Miller SC, Huang R, Sakamuru S, Shukla SJ, Attene-Ramos MS, Shinn P, Van Leer D, Leister W, Austin CP, Xia M (2010) Identification of known drugs that act as inhibitors of NF-kappaB signaling and their mechanism of action. Biochem Pharmacol 79(9):1272–1280. doi:10.1016/j.bcp.2009.12.021
Sonpavde G, Jian W, Liu H, Wu MF, Shen SS, Lerner SP (2009) Sunitinib malate is active against human urothelial carcinoma and enhances the activity of cisplatin in a preclinical model. Urol Oncol 27(4):391–399. doi:10.1016/j.urolonc.2008.03.017
Zwolak P, Jasinski P, Terai K, Gallus NJ, Ericson ME, Clohisy DR, Dudek AZ (2008) Addition of receptor tyrosine kinase inhibitor to radiation increases tumour control in an orthotopic murine model of breast cancer metastasis in bone. Eur J Cancer 44(16):2506–2517. doi:10.1016/j.ejca.2008.07.011
Ikezoe T, Yang Y, Nishioka C, Bandobashi K, Nakatani H, Taguchi T, Koeffler HP, Taguchi H (2006) Effect of SU11248 on gastrointestinal stromal tumor-T1 cells: enhancement of growth inhibition via inhibition of 3-kinase/Akt/mammalian target of rapamycin signaling. Cancer Sci 97(9):945–951. doi:10.1111/j.1349-7006.2006.00263.x
Guerin O, Formento P, Lo Nigro C, Hofman P, Fischel JL, Etienne-Grimaldi MC, Merlano M, Ferrero JM, Milano G (2008) Supra-additive antitumor effect of sunitinib malate (SU11248, Sutent) combined with docetaxel. A new therapeutic perspective in hormone refractory prostate cancer. J Cancer Res Clin Oncol 134(1):51–57. doi:10.1007/s00432-007-0247-4
Yoon CY, Lee JS, Kim BS, Jeong SJ, Hong SK, Byun SS, Lee SE (2011) Sunitinib malate synergistically potentiates anti-tumor effect of gemcitabine in human bladder cancer cells. Korean J Urol 52(1):55–63. doi:10.4111/kju.2011.52.1.55
Ding W, Cai T, Zhu H, Wu R, Tu C, Yang L, Lu W, He Q, Yang B (2010) Synergistic antitumor effect of TRAIL in combination with sunitinib in vitro and in vivo. Cancer Lett 293(2):158–166. doi:10.1016/j.canlet.2010.01.005
Acknowledgments
We thanked Pfizer pharmaceutical company for kindly providing sunitinib compound. This work was supported by the research grant from Taichung Veterans General Hospital (TCVGH-1003701B), National Science Council (NSC99-3112-B-075A-001) and Tunghai University (TCVGH-T1007802).
Author contributions
C-L Teng and S-L Hsu performed the research and wrote the paper, W-L Hwang, C-T Yu and G-Y Hwang designed research and revised the paper, and H-C Liu and J-R Tsai performed the experiments.
Conflict of interest
The authors have no conflicting financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guang-Yuh Hwang and Shih-Lan Hsu contributed equally and are both correspondents to this manuscript.
Rights and permissions
About this article
Cite this article
Teng, CL.J., Yu, CT.R., Hwang, WL. et al. Effector mechanisms of sunitinib-induced G1 cell cycle arrest, differentiation, and apoptosis in human acute myeloid leukaemia HL60 and KG-1 cells. Ann Hematol 92, 301–313 (2013). https://doi.org/10.1007/s00277-012-1627-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-012-1627-7