Abstract
Along with their immunogenic role, dendritic cells (DCs) are also critical in maintaining tolerance to self-antigens by inducing regulatory T cells (Tregs) via the expression of the immunomodulatory enzyme indoleamine 2,3-dioxygenase 1 (IDO1). In turn, Tregs modulate the maturation and/or function of DCs. In immune thrombocytopenia (ITP), the interaction between DCs and Tregs has never been investigated although decreased number/function of Tregs as well as altered DCs have been described. Here, we ask whether, in ITP: (1) IDO1 expression/activity is decreased in mature DCs; (2) IDO1-mediated Treg generation is impaired; and (3) DC maturation is abnormally modulated by Tregs. We found that in ITP, DCs show reduced capability of upregulating the expression/activity of IDO1. This finding results in the reduced ability of mature DCs of converting T cells into Tregs. In turn, Tregs are characterized by decreased interleukin-10 production and show lower ability of inhibiting DC maturation. In conclusion, these data point out the role of IDO1 in the impaired regulatory T cell development of ITP patients and suggest that the cross-talk between Tregs and DCs is hampered and plays a pathogenetic role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regulatory T cells (Tregs) are a subset of T cells involved in the maintenance of peripheral self-tolerance. They suppress immune responses by a mechanism based on a three-way interaction between Tregs and antigen-presenting cells (APCs), T helper (Th) cells, and B cells. Tregs exert their immune control by affecting the functions and numbers of target cells through soluble factors and cell-associated molecules [1–3]. Specifically, Tregs modulate the maturation and/or function of dendritic cells (DCs) [4, 5].
Along with their immunogenic role, DCs are also critical in maintaining tolerance to self-antigens [6]. Indoleamine 2,3-dioxygenase 1 (IDO1) is an immunoregulatory enzyme that catalyzes the rate-limiting step of tryptophan metabolism along the kynurenine pathway and is specifically inhibited by 1-methyltryptophan. IDO1 expression can induce or maintain peripheral immune tolerance [7–9]. In APCs, the upregulation of IDO1, in response to infection or tissue inflammation, inhibits T cell activation and proliferation and induce T cell apoptosis by tryptophan catabolism and deprivation [10]. Moreover, IDO1-expressing DCs have been shown to expand/induce Tregs [11–13]. In turn, Tregs express cytotoxic T lymphocyte antigen-4 (CTLA-4), which interacts with CD80 and CD86 co-stimulatory molecules to trigger the upregulation of IDO1 in DCs resulting in the generation of Tregs and in IDO1-mediated tryptophan catabolism and suppression of T cell activity. Therefore, mature DCs become IDO1 positive (tolerogenic) or remain IDO1 negative (immunogenic) also depending on the influence of Tregs. Among the mediators of tumoral immune escape, IDO1 has recently gained considerable attention [14, 15]. In addition, it has been proposed that IDO-expressing DCs may play a role in preventing the initiation of autoimmune disorders, and impaired IDO-mediated tryptophan catabolism is involved in the development of autoimmune conditions [7, 16].
Immune thrombocytopenia (ITP) is an autoimmune disorder in which, for reasons that remain unclear, platelet surface proteins become antigenic. The activation of the immune system results in autoantibody-mediated platelet destruction and suppression of platelet production. T cell-mediated platelet lysis may also contribute to platelet destruction in ITP [17–19]. APCs, Th cells, and B cells play a crucial role in the pathogenesis of the disease by escaping the immune surveillance exerted by Tregs [20–22]. In addition, the number and/or function of Tregs are impaired in ITP patients [23–27].
Given the crucial role of DCs and Tregs in initiating, regulating, maintaining, or suppressing immune responses, investigations on their interaction are of great importance for better elucidating the pathogenesis of ITP. Therefore, in the present study, we assessed whether in ITP the abnormal interaction of Tregs with DCs might play a pathogenetic role. We demonstrate that in ITP, mature DCs show reduced capability of upregulating the expression/activity of the immune inhibitory enzyme IDO1. This finding results in the decreased ability of DCs of converting non-Treg cells into Tregs. In turn, Tregs show reduced ability to inhibit DC maturation.
Materials and methods
Patients and controls
Twenty-one male and 39 female patients with active ITP, aged between 19 and 72 years (median age 38), were studied. The diagnosis of ITP was made according to Rodeghiero et al. [28] and Provan et al. [29]. At the time of the study, 21 patients were newly diagnosed and 39 patients had persistent or chronic ITP. All patients with persistent (28 cases) or chronic (11 cases) ITP had already received corticosteroid therapy. Specifically, 16 patients with persistent and 6 with chronic ITP were treated with high dose dexamethasone (40 mg/day) given in 4-day pulses every 14 days for three cycles. In addition, 11 patients with persistent and 6 with chronic ITP received prednisone (1 mg/kg/day) for 30 days followed by dose tapering within 20 days. Of note, at the time of sample collection, all patients with persistent or chronic ITP were out of any treatment for at least 2 months. In addition, none of the patients received previous treatment with rituximab or were splenectomized. The median platelet count at the time of the study was 39 × 109/L (range 8–99). A control group of 57 healthy subjects was also enrolled. All subjects (patients/controls) provided a written informed consent for the study. This study was approved by the local medical ethical committee.
Blood sampling and flow cytometry analysis
Peripheral blood mononuclear cells (PBMNCs), anticoagulated with ethylenediaminetetraacetic acid, were isolated from the peripheral blood (PB) (60 mL) of controls and patients via density gradient centrifugation using Ficoll-Hypaque (Cedarlane-Celbio, Milan, Italy). Immunofluorescence was performed using the following panel of monoclonal antibodies (MoAbs): peridin chlorophyll protein-conjugated human anti-CD4, fluorescein isothiocyanate (FITC)-conjugated human anti-CD25, phycoerythrin (PE)-conjugated human anti-CTLA-4, and PE-conjugated human anti-CD119; all from BD Pharmingen (Milan, Italy). Negative controls were isotype-matched irrelevant MoAbs from BD Pharmingen and were used for setting limits of nonspecific immunoglobulin cell binding. The PE-conjugated FoxP3 antibody reagent kit and IgG1 isotype control from Biolegend (San Diego, CA, USA) were used for intracellular FoxP3 staining according to the manufacturer's instructions, using a fixation/permeabilization solution and permeabilization buffer supplied by the same company. Briefly, PBMNCs were stained with combinations of saturating amounts of fluorochrome-conjugated MoAbs for 30 min on ice. After washing with phosphate buffered saline, cells were gated for lymphocytes on the basis of the forward and side scatter profile and analyzed by using a BD FACSCanto II equipment (Bekton Dickinson, Milan, Italy). A minimum of 10,000 events was collected. CD4+CD25high T cells were defined according to previous studies that demonstrated that T cells with regulatory capacity represent, an average, 1–2 % of CD4+CD25+ T cells with the highest expression of CD25 [30]. In vitro-generated Tregs were identified as CD4+CD25highFoxP3+ at flow cytometry.
Isolation of monocytes and generation of DCs
Monocytes were selected with anti-CD14 microbeads (Miltenyi Biotec, Auburn, CA, USA) from PBMNCs and used to generate DCs. Immature (day 5) and mature (day 7) ex vivo-generated DCs were obtained after treatment with lipopolysaccharide (LPS; 1 ng/μL; Sigma-Aldrich, Milan, Italy) for 2 days, as previously described [31].
Isolation of circulating DCs
Circulating DCs were isolated from PBMNCs by blood dendritic cell isolation kit II (Miltenyi Biotec), according to the manufacturer's instructions. This kit allows the multistep concurrent isolation of plasmacytoid DCs (CD304+ cells), myeloid DCs1 (MDC1 and CD1c+ cells), and myeloid DCs2 (MDC2 and CD141+ cells). To achieve the highest purity, the positive fraction was separated over a second column. Purity of the cell population after sorting ranged from 90 to 96 %. Maturation of freshly isolated circulating DCs was obtained as described above and it showed upregulation of the classical maturation markers CD80, CD86, and CD40 (data not shown).
Isolation of highly purified CD4+CD25+ and CD4+CD25− T cell subsets
Circulating CD4+CD25+ and CD4+CD25− T cells were isolated by MiniMACS CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec), according to the manufacturer's instructions. Briefly, CD4+ T cells were isolated from PBMNCs by negative selection. CD4+ T cells were then incubated with mouse anti-human CD25 MicroBeads (2 μL of anti-human CD25 beads/107 CD4+ T cells) and separated into CD4+CD25+ and CD4+CD25− T cells on a positive selection column. To achieve the highest purity, positive and negative cell fractions were separated over a second column. This separation resulted in less than 1 % CD25+ cells in the CD4+CD25− fraction and greater than 97 % purity in the CD25+ subset. In addition, flow cytometry analysis of the isolated CD4+CD25+ T cells demonstrated that these cells were mainly constituted by the CD4+CD25bright cell fraction, which included FoxP3+ cells (Foxp3+ T cells were 90 ± 3 % of the CD4+CD25 bright population; data not shown).
In vitro conversion of CD4+CD25− T cells into Tregs
To evaluate the in vitro conversion of CD4+CD25− T cells into CD4+CD25highFoxP3+ T cells, CD4+CD25− T cells were cultured alone and with autologous mature ex vivo-generated DCs (104/well) from ITP patients or healthy subjects at 10:1 ratio in round-bottom 96-well microtiter plates for 5 days in the presence of RPMI complete medium. All tests were carried out in triplicate. The percentages of CD4+CD25highFoxP3+ T cells were quantitated in the cell suspension at flow cytometry, as above described.
In parallel experiments, to analyze the effect of the inhibition of IDO1 on the in vitro conversion of CD4+CD25− T cells into CD4+CD25highFoxP3+ T cells, CD4+CD25− T cells were cultured alone and with autologous mature ex vivo-generated DCs (104/well) from ITP patients or healthy subjects at 10:1 ratio in the presence or absence of the IDO1-specific inhibitor 1-methyl-l-tryptophan (L-1MT; 1 μM; Sigma-Aldrich). The percentages of inhibition in the generation of CD4+CD25highFoxP3+ T cells were then evaluated by comparing the values of treated/untreated samples.
IDO1 expression/activity in DCs
Total RNA was extracted from immature and mature ex vivo-generated DCs and from circulating DCs, either freshly isolated or after maturation with LPS, of ITP patients and healthy subjects with Qiagen RNeasy microplus kit according to manufacturer's instruction (Qiagen, Valencia, CA, USA). Of RNA, 0.5 μg was used for retro-transcription with ImProm-II™ Reverse Transcriptase (Promega Corporation, Madison, WI, USA) and random hexamers (Invitrogen, Carlsbad, CA, USA), according to manufacturer's instruction (Promega Corporation). Real-time quantitative RT-PCR (qRT-PCR) was performed on an ABI PRISM 7900 Sequence Detector (Perkin Elmer) using SDS2.3 software. qRT-PCR data were analyzed using the 2−ΔΔCt method [32]. The relative level of IDO1 mRNA was calculated by subtracting cycle threshold (Ct) values of the control gene (GAPDH) from the Ct values of the IDO1 gene. Universal human RNA (Stratagene; Agilent Technologies) was used as reference and taken as a value of 1. The IDO1 assay ID is Hs 00175686_ml and GAPDH assay ID is Hs 00266705_gl. All reactions were performed in duplicate.
Kynurenine levels were measured to determine IDO1 enzyme activity, as previously described [14]. Mature ex vivo-generated DCs were harvested, washed, resuspended in Hanks buffered saline solution supplemented with 500 μM l-tryptophan (Sigma-Aldrich), and then incubated for up to 4 h. Supernatants were harvested and kynurenine was detected by a spectrophotometric assay. Briefly, supernatants were mixed with 30 % trichloroacetic acid (Sigma-Aldrich; 2:1), vortexed, and centrifuged at 8,000×g for 5 min. Subsequently, 75 μL of this mixture was added to an equal volume of Ehrlich reagent (AppliChem-VWR International, Milan, Italy) in a 96-well microtiter plate. Triplicate samples were run against a standard curve of defined kynurenine concentrations (6.25–1,000 μM; Sigma-Aldrich). Optical density was measured using a Multiscan EX (M-Medical, Milan, Italy) at 490 nm.
Suppressive activity of in vitro-generated Tregs
To test the suppressive activity of in vitro-generated Tregs, mixed leukocyte reaction (MLR) was performed. Irradiated (3,000 cGy) in vitro-generated CD4+CD25+ T cells (105), which had been isolated as above described, were added to cultures consisting of the same donor-derived CD4+CD25− T cells (105) as responders and stimulated with plate-bound anti-CD3 plus soluble anti-CD28 antibodies (1 μg/mL; BD Biosciences). Cells were then pulsed with 3H-thymidine (1 μCi/well; Amersham Pharmacia Biotech, Piscataway, NJ, USA) for 18 h before harvest on day 5. 3H-thymidine incorporation was measured by scintillation counting. Control wells, containing CD4+CD25+ cells, or CD4+CD25− cells were also monitored. In each assay, all tests were carried out in triplicate. The percentage of inhibition of T cell proliferation was calculated as 1− (cpm incorporated in the co-culture/cpm of responder cells alone) × 100.
Detection of DC maturation after co-cultures with CD4+CD25+/− T cells
Buffy coats were obtained during the preparation of transfusion products from healthy adults. Monocytes were selected with anti-CD14 microbeads from MNCs (after density gradient centrifugation using Ficoll-Hypaque) of buffy coats and used to generate immature DCs, as above described. Therefore, to test the ability of circulating Tregs to inhibit DC maturation, ex vivo-generated immature DCs from buffy coats were cultured alone and with allogeneic CD4+CD25+ T cells from healthy subjects or ITP patients at 1:1 ratio for 2 days in the presence of LPS (1 ng/μL) as stimulator of DC maturation and RPMI complete medium. DCs cultured in the presence of allogeneic CD4+CD25− T cells from healthy subjects or ITP patients were also analyzed for comparison. All tests were carried out in triplicate. The following FITC/PE-conjugated human MoAbs (Becton Dickinson) were used for phenotype characterization of DC maturation: human anti-CD80, human anti-CD86, human anti-HLA-DR, human anti-CD40, and human anti-CD83. Negative controls were isotype-matched irrelevant MoAbs from the same company. Samples were analyzed on a BD FACSCanto II flow cytometer, as previously described [31] and a minimum of 10,000 events was collected.
Cytokine analysis of DCs plus CD4+CD25+/− T cell co-cultures
Immediately prior to DC labeling, the supernatants of ex vivo-generated DCs alone and co-cultured with CD4+CD25+/− T cells were harvested and centrifuged. Supernatants were frozen and stored at −80 °C until further use. The simultaneous measurement of some cytokines in the supernatants was performed using the FlowCytomix Human Th1/Th2 Sample Kit (Bender MedSystem, Vienna, Austria). Specifically, we have evaluated the release of: interleukin-(IL-)1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and TNF-β. Briefly, calibration curves were prepared in sample diluent, with a range of 20.8 to 0.5 pg/mL, dependent on the cytokine under analysis. Twenty-five microliters of standard mixture dilution was added to calibrators then 25 μL bead mixture and 50 μL biotin conjugate mixture were added to calibrators and samples. Each tube was incubated for 2 h at room temperature, protected from light. Following two wash steps, 50 μL streptavidin-PE was added to each tube and incubated for 1 h at room temperature, protected from light. Following two wash steps, each tube was analyzed on BD FACSCanto II equipment. The concentration of each analyte in the array was quantified by comparing the PE intensities for each unknown sample to the corresponding standard curves calculated from the standard sample results by FlowCytomix Pro 2.2 Software (Bender MedSystem).
In parallel experiments, CD4+CD25+ or CD4+CD25− T cells and ex vivo-generated DCs were analyzed after co-culture for the expression of IL-10 using intracellular cytokine staining and flow cytometry analysis. To stop IL-10 release, brefeldin (0.5 μg/μL) was added after 4 h of co-culture. After 24 h of co-cultures, cells were fixed and permeabilized at room temperature. After washing, intracellular staining was next done by adding 20 μL of the PE-conjugated anti-human IL-10 MoAb (BD Biosciences) and incubating at room temperature for 15 min. Cells were gated on the basis of the forward and side scatter profile and were analyzed by using a BD FACSCanto II equipment (Bekton Dickinson). A minimum of 10,000 events was collected. Results were expressed as percentage of IL-10-positive cells.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). Where indicated, differences were compared using the nonparametric Wilcoxon rank sum test. Spearman's rank correlation test was used for correlation analysis. P values <0.05 were considered statistically significant.
Results
Reduced upregulation of IDO1 expression in mature DCs from ITP patients
We first quantified mRNA expression in immature/mature ex vivo-generated DCs from ITP patients and healthy individuals. As expected, IDO1 transcript level was significantly (p < 0.001) higher in mature DCs as compared to immature counterparts both in patients and controls (data not shown). IDO1 expression in immature DCs was comparable between ITP patients and healthy subjects (4.7 ± 3.0 versus 1.9 ± 1.8, respectively; p = not significant (NS)). However, as shown in Fig. 1a, the upregulation of IDO1 expression from immature to mature DCs was significantly reduced in ITP patients as compared with healthy controls (change of 280 ± 44-fold versus 413 ± 56-fold, respectively; p < 0.01).
Reduced upregulation of IDO1 expression in mature DCs from ITP patients. Quantitative RT-PCR of IDO1 expression has been performed on a immature/mature ex vivo-generated DCs and on b freshly isolated/mature circulating DCs from 14 ITP patients and 15 healthy subjects. IDO1 upregulation, expressed as fold-change of 2−ΔΔCt mature/immature DCs, was significantly reduced either in ex vivo-generated DCs (*p < 0.01) or in circulating DCs (*p < 0.05) from ITP patients in comparison with the normal counterparts. c Supernatants from equal number of mature ex vivo-generated DCs were resuspended in tryptophan-enriched medium and tested after 4 h by a spectrophotometric assay for production of the tryptophan catabolite, kynurenine, as an index of IDO activity. Kynurenine concentration was significantly (*p < 0.05) lower in the supernatant from ITP patients as compared with healthy subjects. Results are expressed as mean ± SD
In a second set of experiments, we evaluated IDO1 in circulating DCs before and after maturation with LPS. Once again, IDO1 expression in circulating DCs was comparable between healthy subjects and ITP patients (10.4 ± 2.1 versus 13.1 ± 6.8, respectively). However, consistent with ex vivo-generated DCs, the upregulation of IDO1 in PB DCs after maturation was significantly reduced in ITP patients as compared with healthy individuals (change of 36 ± 4-fold versus 45 ± 3-fold, respectively; p < 0.05 (Fig. 1b)). Of note, we did not observe any differences in the quantitative expression of IDO1 transcripts between newly diagnosed versus persistent or chronic ITP (data not shown).
Accordingly, when we evaluated kynurenine levels as an index of IDO1 enzyme activity, we found that kynurenine concentration in the supernatants from equal numbers of mature DCs was significantly lower in ITP patients (40.7 ± 12.5 μM; eight cases) as compared to healthy subjects (66.5 ± 9.4 μM; ten cases; p < 0.05) (Fig. 1c). These data demonstrate that in ITP, the upregulation of IDO1 expression in mature DCs, either ex vivo-generated or circulating, is defective.
Defective in vitro conversion of CD4+CD25− T cells into Tregs by mature DCs from ITP patients
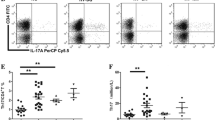
According to previous results [23–27], we found that in ITP patients, the absolute number and the suppressive function of circulating Tregs were significantly (p < 0.02 and p < 0.05, respectively) lower than that observed in healthy subjects, with a positive correlation between the number of circulating CD4+CD25highFoxP3+ T cells and the platelet count (r, 0.4; p < 0.02) (Supplementary Figs. 1 and 2).
Therefore, based on these findings and on the well-known relationship between the upregulation of IDO1 and Treg induction, an additional set of experiments was performed to assess whether in ITP, DCs, which have IDO1-reduced expression and activity (Fig. 1), are less efficient in generating Tregs in vitro. We characterized the in vitro conversion of highly purified circulating CD4+CD25− T cells into CD4+CD25highFoxP3+ T cells after stimulation with mature ex vivo-generated DCs. Figure 2a shows the representative expression of FoxP3 in in vitro-generated CD4+CD25high T cells. After co-cultures of DCs from healthy subjects or ITP patients with autologous CD4+CD25− T cells, the mean percentage of FoxP3+ cells (gated on CD4+CD25high T cells) was significantly reduced in ITP patients (54.4 ± 4.5 %) in comparison with healthy individuals (88.3 ± 5.8 %; p < 0.01) (Fig. 2b). Also in this case, we did not observe any difference in the number of in vitro-generated Tregs between newly diagnosed versus persistent or chronic ITP (data not shown). Importantly, as shown in Fig. 2c, the addition of the IDO1-specific inhibitor L-1MT to co-cultures significantly (p < 0.02) inhibited the generation of CD4+CD25highFoxP3+ T cells in ITP patients. Thus, we confirm that, similar to normal donors, in ITP, CD4+CD25+ T cell induction by conversion of CD4+CD25− T cells may be due, at least in part, to IDO1 activity.
Defective in vitro conversion of CD4+CD25− T cells into Tregs by mature DCs from ITP patients. To test the in vitro conversion of non-Tregs into Tregs, mature ex vivo-generated DCs from eight ITP patients or eight healthy subjects were co-incubated with highly purified autologous CD4+CD25− T cells for 5 days at 1:10 ratio. Tregs were identified at flow cytometry as FoxP3+ cells of CD4+CD25high T cells. a One representative experiment of the flow cytometric identification of in vitro-generated normal FoxP3+ cells of CD4+CD25high T cells is shown. Gate P3 identifies CD4+CD25high T cells. Gate P4 shows FoxP3+ cells of CD4+CD25high T cells. b The percentage of in vitro-generated Tregs was significantly (*p < 0.01) reduced in ITP patients as compared with healthy subjects. c Mature ex vivo-generated DCs from six ITP patients were co-incubated with autologous CD4+CD25− cells at 1:10 ratio and in presence or absence of L-1MT. The addition of L-1MT to co-cultures significantly (*p < 0.02) reduced the generation of Tregs. The results are expressed as percentage of positive cells. d Suppressive activity of in vitro-generated Tregs (MLR). Irradiated CD4+CD25+ T cells (105), which had been obtained after in vitro conversion of CD4+CD25− T cells by mature DCs and immunomagnetic isolation, were added to cultures of autologous CD4+CD25− T cells (105) as responders and stimulated with anti-CD3 plus anti-CD28 antibodies. The degree of proliferation was assessed by incorporation of 3H-thymidine. The percentage of inhibition of proliferation of CD4+CD25− T cells from four patients with ITP and five healthy subjects is shown. The in vitro-generated Tregs of ITP patients show reduced suppressive activity. (*p < 0.04). Results in b–d are expressed as mean ± SD
We then assessed whether these in vitro-generated CD4+CD25+ T cells possess regulatory activity. Therefore, CD4+CD25+ T cells, either from ITP patients or healthy subjects, were immuno-magnetically isolated and tested for their ability of inhibiting the proliferation of CD4+CD25− T cells. As shown in Fig. 2d, the proliferative response of CD4+CD25− cells from controls in co-culture with autologous in vitro-generated CD4+CD25+ T cells was inhibited by 52 ± 6 %. In ITP patients, CD4+CD25+ T cells were less effective suppressors by inhibiting the proliferation of autologous CD4+CD25− cells by 29 ± 5 % (p < 0.04).
Taken together, these results show that in ITP: (1) IDO1 enzyme is functionally active in mature DCs; however, (2) DC-mediated in vitro generation of Tregs is defective because IDO1 expression is decreased; thus, (3) T cell proliferation inhibition induced by Tregs is reduced.
Reduced ability of highly purified circulating Tregs to modulate DC maturation in ITP patients
To evaluate whether Tregs induce functional differences in DCs, we first assessed the production of cytokines in the supernatants of normal immature DCs co-cultured with highly purified circulating CD4+CD25+ or CD4+CD25− T cells from ITP patients or healthy subjects. Increased IFN-γ and IL-2 levels were observed in the supernatant of DCs plus CD4+CD25− T cells of ITP patients as compared with the normal counterparts. Conversely, no significant differences were observed in the concentration of the following T cell-derived cytokines (IL-2, IL-4, IL-5, tumor necrosis factor-α, transforming growth factor-β, and IFN-γ) and DC-associated cytokines (IL-1, IL-12, and IL-8) in the supernatant of DCs plus CD4+CD25+ T cells between patients and controls (data not shown). However, the concentration of IL-6 and IL-10 was significantly reduced in the supernatant of DCs plus CD4+CD25+ T cells of ITP patients (690 ± 145 versus 1.119 ± 112 pg/mL in normal donors for IL-6 and 99 ± 26 versus 218 ± 19 pg/mL for IL-10) (p < 0.01; Fig. 3a, b).
Reduced amount of IL-10 and IL-6 in the supernatants of DC-T cell co-cultures from ITP patients. Normal immature ex vivo-generated DCs were co-cultured alone and with allogeneic CD4+CD25+ and CD4+CD25− T cells, either from six ITP patients or six healthy subjects at 1:1 ratio. IL-6 and IL-10 were measured in the supernatants by an ELISA kit. The supernatant of DCs co-cultured with the CD4+CD25+ T cells from ITP patients showed significant (*p < 0.01) reduced level of IL-6 a and IL-10 b as compared with the normal counterpart. The IL-6 and IL-10 mean values of the co-cultures of DCs plus CD4+CD25− T cells were not significantly different between patients and controls. Results are expressed as mean ± SD
Since IL-10 plays a central role in immune tolerance and can be produced by either DCs or T cells [33], we performed the intracytoplasmic staining of the cytokine to evaluate whether its secretion was due to DCs upon co-culture and/or produced by Tregs. The mean percentage of IL-10-producing Tregs was significantly (p < 0.04) lower in ITP patients (19.3 ± 8.0 %) in comparison with that of normal individuals (34.9 ± 6.6 %). By contrast, the percentage of IL-10-producing DCs after co-culture with CD4+CD25+ T cells was comparable between patients and controls (Fig. 4a, b).
Defective IL-10-producing Tregs after co-cultures with DCs in ITP patients. a Intracytoplasmic IL-10 content determined by flow cytometry of ex vivo-generated DC-T cell co-cultures. Histogram analysis of IL-10 content of DCs (red histogram) and CD4+CD25+ or CD4+CD25− T cells (green histogram) after co-cultures is shown as representative experiments in one healthy subject and one ITP patient. Gate P3 identifies the percentage of IL-10-producing Tregs. b The bar graph represents the mean percentage of IL-10 contents of Tregs from five ITP patients and four healthy subjects. Results shown are mean ± SD of all experiments and significant (*p < 0.04) difference is indicated
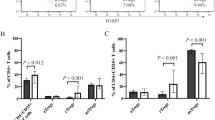
We then analyzed the capacity of highly purified circulating Tregs of inhibiting DC maturation in ITP patients and healthy individuals. We demonstrated that when normal immature DCs were co-cultured with normal CD4+CD25+ T cells, the expression of the co-stimulatory molecules CD86 (Fig. 5a) and CD80 (Fig. 5b) was significantly lower compared with DCs cultured in the presence of CD4+CD25− T cells (p < 0.05). Conversely, CD4+CD25+ T cells from ITP patients failed to inhibit the expression of CD80 and CD86 molecules on DCs (Fig. 5a, b). In addition, when we compared the CD86 and CD80 expression of DCs after co-cultures with CD4+CD25+ T cells, we found no significant differences between ITP patients and healthy subjects (p = NS).
Reduced capability of highly purified circulating Tregs from ITP patients of inhibiting DC maturation. Immature ex vivo-generated DCs from healthy individuals were co-cultured alone and with allogeneic CD4+CD25+ or CD4+CD25− T cells from six healthy subjects or six ITP patients at 1:1 ratio for 2 days in the presence of LPS as stimulator of DC maturation. a CD86 expression. Ratios between mean intensity fluorescence (MIF) of DCs plus CD4+CD25+ or CD4+CD25− T cells and MIF of DCs alone are shown. CD4+CD25+ T cells from healthy subjects significantly (*p < 0.05) inhibited CD86 expression of DCs in comparison with the CD4+CD25− T cells. At variance, CD4+CD25+ T cells from ITP patients failed to inhibit CD86 expression on DCs. b CD80 expression. Ratios between MIF of DCs plus CD4+CD25+ or CD4+CD25− T cells and MIF of DCs alone are shown. The same pattern was observed when the expression of CD80 was monitored (*p < 0.05). Results are expressed as mean ± SD
CD40, HLA-DR, and CD83 expression on DCs was unaffected by Treg-DC co-cultures either in patients or in controls. Of note, we did not observe any differences between newly diagnosed versus persistent or chronic ITP (data not shown).
Taken together, these data provide additional evidence that in ITP, Tregs are functionally defective because they do not prevent DC maturation.
Discussion
Recent evidence suggests that there is a well-defined interplay between the two cell populations (Tregs and DCs) regulating effector T cells [2, 34, 35]. This interaction may result in the induction of immunological tolerance. In fact, the concept that “immature DCs are tolerogenic whereas mature DCs are immunogenic” has been recently challenged by several studies showing that fully mature DCs can induce tolerance. Specifically, it has been demonstrated that mature DCs expand autologous/allogeneic Tregs by an IDO1-dependent mechanism [2, 6, 7]. On the other hand, previous studies documented that Tregs are able to force DCs to acquire a tolerogenic phenotype and function [4, 5, 36–38]. Tregs inhibit the maturation process and antigen presentation capacity of DCs by downregulating the expression of co-stimulatory molecules and upregulating soluble factors like the anti-inflammatory IL-10.
ITP is an autoimmune disorder in which, for reasons that remain unclear, platelet surface proteins become antigenic and stimulate the immune system to produce autoantibodies and self-reactive cytotoxic T lymphocytes. These findings result in immune-induced platelet destruction and suppression of platelet production [17–19].
Few cellular mechanism(s) of failure of immune tolerance in ITP has been investigated by many authors [20, 23–27, 39, 40]. Prior data showed that in ITP platelet, glycoprotein-specific-induced Tregs can be generated de novo from non-regulatory CD4+CD25−CD45RA+ cells and glycoprotein-induced Treg-modulated DCs display a semi-mature phenotype [41]. However, the authors did not report data comparing patients and controls and did not characterize the mechanism of Treg generation. Furthermore, a study by Wang et al. [42] demonstrated that the plasma kynurenine concentration is significantly reduced in ITP patients. In addition, in CD4+ and CD8+ T cells of the ITP patients, but not in CD19+ and CD14+ cells, IDO expression is lower than that in healthy controls. Very recently, Xu et al. [43] demonstrated that monocyte-derived DCs from ITP patients show decreased IDO1 expression and that CTLA-4-Ig, as previously described [44], induces IDO1+ expression in monocyte-derived DCs, which, in turn, increase the percentage of CD4+CD25+FoxP3+ T cells. However, despite these findings, no data have been published on the role played by the IDO1-mediated interaction between Tregs and DCs in the pathogenesis of ITP.
In the present study, for the first time, we studied the bidirectional functional effects of DC and Treg interplay in ITP. We demonstrate that in ITP, ex vivo expanded as well as freshly isolated circulating DCs show decreased ability of upregulating IDO1expression/activity after in vitro maturation with LPS. This finding results in the reduced ability of mature DCs of converting non-Treg cells into Tregs, since DCs expressing IDO1 favor Tregs. Whether in vitro-altered conversion of non-Tregs into Tregs found in our patients with ITP mirrors in vivo-disturbed generation of Tregs remains a matter of speculation. However, our results suggest that in ITP, the IDO1-dependent mechanism of Tregs generation is impaired and may play a pathogenetic role in regulating the frequency of circulating Tregs.
In this view, it has recently been demonstrated that IDO1 inhibition can increase CD86 expression and consequently may promote the ability of DCs to activate T cells [45]. Consistently, we previously found that in ITP patients, DCs show upregulated expression of the co-stimulatory molecule CD86 [31]. Therefore, in ITP, the increased expression of CD86 on DCs may be due, at least in part, to the reduced expression of IDO1.
Interestingly, our study demonstrates that the in vitro addition of the IDO1-specific inhibitor L-1MT to co-cultures did not totally abrogated the generation of CD4+CD25highFoxP3+ T cells. This finding suggests that other mechanisms may be relevant in inducing this T cell population. Factors that promote Treg cell phenotype (that is TGF-β) may play a role also [46]. Furthermore, since IDO1 expression can be modulated by IFN-γ in DCs [47] and circulating IFN-γ is increased in patients with chronic ITP [48, 49], we evaluated therefore whether IFN-γ receptor (CD119) expression was decreased in mature ex vivo-generated DCs of ITP patients. However, we did not observe any difference between patients and controls (data not shown). Thus, we cannot conclude that impaired IFN-γ receptor expression plays a significant role in the defective upregulation of IDO1 in DCs from ITP patients. Moreover, we cannot exclude that in ITP, alternative mechanisms may be involved. It can be hypothesized that Tregs are destroyed or consumed in an attempt to counteract/control the immune response against self-platelet antigens. To support this hypothesis, Treg count/function improves after therapy [24, 27, 50].
In addition, here we show that in ITP in vitro-generated Tregs have reduced suppressive activity. It is likely that this dysfunction is specific to Tregs since a prior study documents that in ITP, the impaired suppression of circulating Tregs is due to a decrease in Treg function and not to refractoriness of CD4+CD25− T cells to suppression [26].
On the other hand, we also demonstrate that in ITP, Tregs show reduced ability to inhibit DC maturation. Tregs, but not DCs, show deficient expression of IL-10, a cytokine that actively suppresses DC maturation. IL-10 is a regulatory cytokine involved in tolerance and IL-10-secreting T cells may constitute an additional mechanism that is responsible for peripheral tolerance [49, 51]. Moreover, our previous data showed that IL-6 is a potent inhibitor of DC development from CD34+ stem/progenitor cells [52, 53]. Therefore, our results showing decreased IL-6 secretion in ITP suggest that IL-6-dependent inhibition of DC maturation may be also defective.
In conclusion, these data point out the role of IDO1 in the impaired regulatory T cell development of ITP patients. In addition, Tregs are functionally defective because they do not prevent DC maturation. As a consequence, DCs are less “tolerogenic” and Tregs are defective in their regulatory function. Therefore, in ITP, the cross-talk between Tregs and DCs is hampered and plays a pathogenetic role. Understanding these mechanisms is essential for the discovery of potential therapeutic targets.
References
Corthay A (2009) How do regulatory T cells work. Scand J Immunol 70:326–336
Wing K, Sakaguchi S (2010) Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol 11:7–13
Campbell DJ, Koch MA (2011) Phenotypical and functional specialization of FoxP3+ regulatory T cells. Nat Rev Immunol 11:119–130
Cederbom L, Hall H, Ivars F (2000) CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol 30:1538–1543
Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S (2008) FoxP3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Nat Acad Sci USA 105:10113–10118
Maldonado RA, von Andrian UH (2010) How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol 108:111–165
Mellor AL, Munn DH (1999) Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation. Immunol Today 20:469–473
Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ (2011) Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases. Curr Med Chem 18:2257–2262
Orabona C, Grohmann U (2011) Indoleamine 2,3-dioxygenase and regulatory function: tryptophan starvation and beyond. Methods Mol Biol 677:269–280
Hill M, Tanguy-Royer S, Royer P, Chauveau C, Asghar K, Tesson L, Lavainne F, Rémy S, Brion R, Hubert FX et al (2007) IDO expands human CD4+CD25 high regulatory cells by promoting maturation of LPS-treated dendritic cells. Eur J Immunol 37:3054–3062
Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC et al (2002) CTLA-4 Ig regulates tryptophan catabolism in vivo. Nat Immunol 3:1097–1101
Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, Young JW (2009) Indoleamine 2,3 dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 114:555–563
Trabanelli S, Ocadlikova D, Evangelisti C, Parisi S, Curti A (2011) Induction of regulatory T cells by dendritic cells through indoleamine 2,3-dioxygenase: a potent mechanism of acquired peripheral tolerance. Curr Med Chem 18:2234–2239
Curti A, Trabanelli S, Onofri C, Aluigi M, Salvestrini V, Ocadlikova D, Curti EC, Rutella S, De Cristofaro R, Ottaviani E et al (2010) Indoleamine 2,3 dioxygenase-expressing leukemic dendritic cells impair leukemia specific immune response by inducing potent T regulatory cells. Haematologica 95:2022–2030
Munn DH (2011) Indoleamine 2,3-dioxygenase, Tregs and cancer. Curr Med Chem 18:2240–2246
Oertelt-Prigione S, Mao TK, Selmi C, Tsuneyama K, Ansari AA, Coppel RL, Coppel RL, Invernizzi P, Podda M, Gershwin ME (2008) Impaired indoleamine 2,3 dioxygenase production contributes to the development of autoimmunity in primary biliary cirrhosis. Autoimmunity 41:92–99
McMillan R (2007) The pathogenesis of chronic immune thrombocytopenic purpura. Sem Hematol 44(suppl 5):S3–S11
Cines DB, Bussel JB, Liebman HA, Luning Prack ET (2009) The ITP syndrome: pathogenic and clinical diversity. Blood 113:6511–6521
Stasi R, Newland AC (2011) ITP: a historical perspective. Br J Haematol 153:437–450
Kuwana M, Ikeda Y (2005) The role of autoreactive T-cells in the pathogenesis of idiopathic thrombocytopenic purpura. Int J Hematol 81:106–112
Andre S, Tough DF, Lacroix-Desmazes S, Kaveri SV, Bayry J (2009) Surveillance of antigen-presenting cells by CD4+CD25+ regulatory T cells in autoimmunity. Am J Pathol 174:1575–1587
Semple JW, Provan D, Garvey MB, Freedman J (2010) Recent progress in understanding the pathogenesis of immune thrombocytopenia. Curr Opin Hematol 17:590–595
Sakakura M, Wada H, Tawara I, Nobori T, Sugiyama T, Sagawa N, Sakakura SH (2007) Reduced CD4+CD25+ T cells in patients with idiopathic thrombocytopenic purpura. Thromb Res 120:187–193
Ling Y, Cao X, Yu Z, Ruan C (2007) Circulating dendritic cells subsets and CD4+FoxP3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high dose dexamethasome. Eur J Haematol 79:310–316
Liu B, Zhao H, Poon MC, Han Z, Gu D, Xu M, Jia H, Yang R, Han ZC (2007) Abnormality of CD4+CD25+ regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol 78:139–143
Yu J, Heck S, Patel V, Levan J, Yu Y, Bussel JB, Yazdanbakhsh K (2008) Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood 112:1325–1328
Stasi R, Cooper N, Del Poeta G, Stipa E, Laura Evangelista M, Abruzzese E, Amadori S (2008) Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood 112:1147–1150
Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113:2386–2393
Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B et al (2010) International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 115:168–186
Baecher Allan C, Brown JA, Freeman GJ, Hafler DA (2001) CD4+CD25 high regulatory cells in human peripheral blood. J Immunol 167:1245–1253
Catani L, Fagioli ME, Tazzari PL, Ricci F, Curti A, Rovito M, Preda P, Chirumbolo G, Amabile M, Lemoli RM et al (2006) Dendritic cells of immune thrombocytopenic purpura (ITP) show increased capacity to present apoptotic platelets to T lymphocytes. Exp Hematol 34:879–884
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J (2010) Biology of interleukin-10. Cytokine Growth Factor Rev 21:331–344
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 30:775–787
Cyetanovich GL, Hafler DA (2010) Human regulatory T cells in autoimmune diseases. Curr Opin Immunol 22:753–760
Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Tadokoro LJJ, Dustin ML (2006) Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med 203:505–511
Veldohen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B (2006) Modulation of dendritic cell function by naïve and regulatory CD4+ T cells. J Immunol 176:6202–6210
Bayry J, Triebel F, Kaveri SV, Tough DF (2007) Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells. J Immunol 178:4184–4193
Kuwana M, Okazaki Y, Ikeda Y (2009) Splenic macrophages maintain autoimmune response via uptake of opsonised platelets in patients with immune thrombocytopenic purpura. J Thromb Haemost 7:322–329
Catani L, Sollazzo D, Ricci F, Polverelli N, Palandri F, Baccarani M, Catani L, Vianelli N, Lemoli RM (2011) The CD47 pathway is deregulated in human immune thrombocytopenia. Exp Hematol 39:486–494
Zhang XL, Peng J, Sun JZ, Liu JJ, Guo CS, Wang ZG, Yu Y, Shi Y, Qin P, Li SG et al (2009) De novo induction of platelet-specific CD4+CD25+ regulatory T cells from CD4+CD25-cells in patients with idiopathic thrombocytopenic purpura. Blood 113:2568–2577
Wang CY, Shi Y, Min YN, Zhu XJ, Guo CS, Peng J, Dong XY, Qin P, Sun JZ, Hou M (2011) Decreased IDO activity and increased TTS expression break immune tolerance in patients with immune thrombocytopenia. J Clin Immunol 31:643–649
Xu S, Wang C, Zhu X, Dong X, Shi Y, Peng J, Qin P, Sun J, Guo C, Ni H, et al. (2012) Decreased indoleamine 2,3-dioxygenase expression in dendritic cells and role of indoleamine 2,3-dioxygenase-expressing dendritic cells in immune thrombocytopenia. Ann Hematol. doi:10.1007/s00277/012-1451-0
Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM (2005) Regulation of Indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood 105:1574–1581
Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M et al (2010) Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 115:3520–3530
Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA (2004) Natural and induced CD4+CD25+ cells educate CD4+CD25-cells to develop suppressive activity: the role of IL-2, TGf-beta and IL-10. J Immunol 172:5213–5221
Taylor MW, Feng GS (1991) Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 5:2516–2522
Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, Freedman J (1996) Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood 87:4245–4254
Panitsas FP, Theodoropoulou M, Kouraklis A, Karakantza M, Theodorou GL, Zoumbos NC et al (2004) Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood 103:2645–2647
Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, Yazdanbakhsh K et al (2010) Improved regulatory T cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 116:326–336
Fujio K, Okamura T, Yamamoto K (2010) The family of IL-10-secreting CD4+ T cells. Adv Immunol 105:99–130
Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Fogli M, Ferri E, Della Cuna GR, Tura S et al (2002) Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood 100:230–237
Frick J-S, Grunebach F, Autenrieth IB (2010) Immunomodulation by semi-mature dendritic cells: a novel role of Toll-like receptors and interleukin-6. Int J Med Microbiol 300:19–24
Acknowledgments
This study was supported by BolognAIL and RFO of the University of Bologna (L.C. 2010, 2011).
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 124 kb)
Rights and permissions
About this article
Cite this article
Catani, L., Sollazzo, D., Trabanelli, S. et al. Decreased expression of indoleamine 2,3-dioxygenase 1 in dendritic cells contributes to impaired regulatory T cell development in immune thrombocytopenia. Ann Hematol 92, 67–78 (2013). https://doi.org/10.1007/s00277-012-1556-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-012-1556-5