Abstract
We aimed to determine the frequency of asymptomatic brain lesions in a group of patients with β-thalassemia intermedia (β-TI) and to evaluate correlation of asymptomatic brain lesions with splenectomy, thrombocytosis, blood transfusions, and clinical parameters. Ninety five neurologically intact patients with β-TI were randomly enrolled in this cross-sectional study. Diffusion-weighted imaging brain MRI was performed in every patient to detect cerebral white matter lesions (WML). We found an overall frequency of 15 (15.8 %) for WMLs, 14 (23.7 %) in splenectomized, and 1 (2.8 %) in nonsplenectomized patients. The presence of WML was significantly associated with splenectomy (P = 0.008) and thrombocytosis (P = 0.009). However, after adjustment for splenectomy, thrombocytosis was not significantly associated with the presence of WML (P > 0.05). The number of patients with regular blood transfusions and normal MRI was not significantly higher compared to those with abnormal findings (52.5 % vs. 26.7 %; P = 0.092). In untransfused patients, hydroxyurea (HU) administration was associated with a lower incidence of WML (P < 0.001). Although in univariate analysis either splenectomy or thrombocytosis showed significant correlation with the presence of single or multiple WMLs, thrombocytosis by itself did not significantly contribute in developing asymptomatic brain lesions. The lack of significant correlation between lesions and regular blood transfusions could be related to the treatment with HU in untransfused patients, which increased fetal hemoglobin levels and improved the morphology and the pathological indices of the red blood cells. Larger prospective studies are suggested for the accurate evaluation of the correlation of these factors with developing asymptomatic brain lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with homozygous forms of thalassemia, the availability of improved medical management has led to increased life expectancy and consequently new complications in thalassemic patients [1, 2] particularly in patients with β-thalassemia intermedia (β-TI) who have milder clinical symptoms compared to patients with β-thalassemia major [3]. However, they develop chronic hypercoagulable state with higher frequency of thromboembolic (TE) complications [4]. Suggested causative mechanisms for chronic hypercoagulable state in thalassemic patients are: increased platelet aggregation, decreased platelet survival [1, 5, 6], decrease in antithrombotic factors including protein C and S [7–10], and lower antithrombin III levels [11, 12]. Splenectomized patients with β-TI [4] have more circulating damaged red blood cells (RBC) resulting in expressing high levels of negatively charged phospholipids, mainly phosphatidylserine, which stimulate thrombin generation [13, 14] with a higher risk for developing TE in different body organs [15]. Cerebral TE events also have been observed in these patients with a higher frequency than normal population. We reported previously a frequency of 28 % of asymptomatic white matter changes in 30 patients with β-TI who had been splenectomized with a platelet count >500,000/μL in southern Iran [16]. Until now, the reported brain abnormalities in patients with β-TI were in relatively small sample sizes [17, 18]. The present study on the incidence of brain abnormalities was extended and carried out in 95 patients with β-TI. In addition, following the report of Taher et al. [19] in 30 adult splenectomized patients on the probable protective effect of regular blood transfusions on the occurrence of asymptomatic cerebral white matter lesions (WML), we evaluated the correlation of the incidence of WML with thrombocytosis, splenectomy, transfusion requirements and parameters such as ferritin level, hemoglobin, sex, age, and hydroxyurea (HU) consumption.
Materials and methods
In this cross-sectional study we randomly selected 95 neurologically intact patients with β-thalassemia intermedia (β-TI) from a total of 240 thalassemic patients referred to the thalassemia center of Shiraz University of Medical Sciences, Southern Iran from October 2010 to June 2011. The diagnosis of β-TI was made by complete blood, RBC indices, Hb level ≥7 g/dL, and Hb electrophoresis. The age at diagnosis and their transfusion requirements were after 2 years old in all patients. The patients were divided into two major groups according to their transfusion requirements; (1) those who are transfusion-independent or receive blood transfusions one to two times a year, and (2) those who receive blood transfusions every 3–4 weeks from the age of 6.4 ± 4.2 years. A data gathering form was designed to obtain all the above-mentioned laboratory parameters in addition to serum ferritin levels, medications including HU, presence or absence of the spleen and family history. The patients were evaluated by an expert neurologist to exclude any signs or symptoms indicating neurological deficit. None of them had any thromboembolic events. Patients who had risk factors of thrombophilia were excluded from the study (evidence of prothrombin, factor V Leiden or MTHFR mutations documented by genetic studies; as well as positive result of antithrombin III, protein C, protein S, lupus anticoagulant, or cardiolipin antibodies).
The study protocol was approved by the Ethics Committee of the Shiraz University of Medical Sciences and informed written consent was obtained from the patients or their families.
The detailed cerebral MRI imaging protocol has been reported elsewhere [20]. Briefly, the imaging protocol included: a sagittal T1-weighted spin echo sequence; an axial fluid-attenuated inversion recovery sequence; an axial T2-weighted fast spin echo sequence and a diffusion-weighted sequence. The MRI has been interpreted by an experienced neuroradiologist for all participants using a 1.5 T MR unit (Siemens, Avanto, Germany) using a standard four-channel head coil. White matter lesions were classified into two groups including ischemia and infarction. These lesions were classified based on size, number of lesions and locations. In patients with multiple lesions, the size of the largest lesion was considered in statistical analysis. In patients with WMLs, other abnormal findings including generalized brain atrophy, hyper signal changes in basal ganglia, midbrain and thalamus, and skull thickening were evaluated and reported. Brain atrophy was determined based on decrease in brain volume more than what expected in a healthy age-matched control and graded as mild, moderate and severe.
Statistical analysis
Data were analyzed by SPSS v. 17 (SPSS Inc., Chicago, IL, USA). Descriptive data were presented as mean ± standard deviation and frequency. Comparison of the characteristics and laboratory data between the two groups of patients with and without MRI findings were done by independent samples t test and Mann–Whitney U test for continuous variables and Fisher’s exact test for qualitative variables. Two-sided P value less than 0.05 was considered statistically significant. Spearman correlation test was done to determine the correlation between two quantitative variables in different subgroups of patients. P value less than 0.05 was considered statistically significant.
Results
Overall, we had 46 transfusion dependent and 49 transfusion-independent patients. Characteristics of the patients presented in Table 1. Their mean age was 23.1 ± 8.2 (range, 6–58 years) including 32 males and 63 females. Positive family history of cerebrovascular disease was reported in two patients and positive family history of TE event was reported in one patient. Of 49 patients who were transfusion-independent, 44 patients (90 %) were treated with HU (mean, 10 mg/kg/day, range of 8–15 mg/kg/day) for duration of 4–13 years. In this subgroup of patients (transfusion-independent), total HbF was increased from 6.3 ± 1.2 to 8.05 ± 2.3 g/dL (P < 0.001) at the end of study. Mean corpuscular volume and mean corpuscular hemoglobin were also increased from 70.6 ± 8.7 to73.5 ± 9.5 and from 23.6 ± 3.1 to 24.8 ± 3.5 respectively. Red blood cell morphology was evaluated before and after 1 year of treatment with HU and showed decreased poikilocytosis in the peripheral blood smears documented by the number of schistocytosis and target cells.
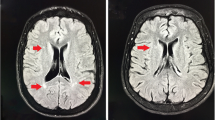
Fifteen (15.8 %) patients had WMLs, 13 with ischemia, and 2 with small infarctions (Fig. 1). Two patients had mild generalized atrophy. Hyper signal changes were observed in these patients as follows: seven patients in basal ganglia, six patients in midbrain, and one patient in thalamus. Moderate and severe skull thickening were observed in seven and five patients, respectively. The distribution of size, number, and location of WMLs are summarized in Table 2. Nine patients (60 %) had evidence of multiple lesions with a mean of 14.2 ± 14.7 and range of 2–50 lesions. Eighty percent of the lesions were between 0.5 and 1 cm (mean, 0.94 ± 0.4 cm; range, 0.2–2 cm) and mainly (46.6 %) located in parietal region.
The results of univariate analysis of covariates in patients with and without brain lesions are presented in Table 3. The presence of abnormal findings in MRI in favor of ischemia or infarction was significantly associated with thrombocytosis (platelet >500,000/μL; P = 0.009) and splenectomy (P = 0.008). Among the group with normal MRI, a higher number of patients received regular blood transfusions compared to patients with abnormal findings (52.5 % vs. 26.7 %); however, it was not statistically significant (P = 0.092). In splenectomized patients, the mean duration since splenectomy was higher in patients with abnormal MRI finding compared to the patients with normal MRI but it was not statistically significant (13.6 ± 10.6 vs. 10.5 ± 6.4, P = 0.360). There was no statistically significant association (P > 0.05) between the frequency of patients with brain lesions and either thrombocytosis or receiving regular blood transfusion, and treatment with HU in splenectomized and nonsplenectomized patients (Table 4).
Patients with WMLs were classified into two groups with either single or multiple lesions. And there were no statistically significant differences between them with regard to thrombocytosis, splencetomy, HU treatment, or regular blood transfusion (P > 0.05). Also, the mean size of the lesions was not significantly correlated with thrombocytosis, splencetomy, HU taking or regular blood transfusion (P > 0.05). Size and number of the lesions were not significantly correlated with age in patients with WMLs (P = 0.782 and P = 0.078, respectively). In splenectomized patients with abnormal MRI finding, duration since splenectomy did not significantly correlated with size (r s = −0.067, P = 0.836) or number of WMLs (r s = −0.141, P = 0.662). There was no significant association between the presence of other abnormal findings with any of the studied variables.
As HU consumption was related to transfusion-independent patients, we conducted a comparison between HU consumption and the presence of WML in this subgroup of patients (49 patients). A lower incidence (6 out of 44, 13.6 %) of WMLs was found in patients who received HU compared to five out of five patients who did not receive HU (P < 0.001).
There were no significant correlations between the presence of WML in MRI and each of the other investigated variables including age, sex, hemoglobin level, and serum ferritin.
Discussion
In this study, we performed DWI brain MRI screening in a large series of β-TI patients without any neurological sign or symptoms. We found an overall frequency of 15 (15.8 %) of WMLs including 14 (23.7 %) in splenectomized, and 1 (2.8 %) in nonsplenectomized patients. In our previous publication, we reported an incidence of 28 % with asymptomatic white matter changes detected by MRI in 30 splenectomized β-TI patients with platelet count >500,000/μL [16]. In the present study, a similar rate (25 %) of WML was found among 48 splenectomized patients with platelet count >500,000/μL, including 30 already reported patients [16]. Taher et al. [19] reported a higher frequency of WMLs in 60 % (18 out of 30) of adult splenectomized patients with β-TI. The relatively lower incidence of WMLs in our patients compared with the patients from Lebanon may be related to their younger age (mean age 25.3 ± 8.3 vs. 32.1 ± 11), as well as possible differences in genetic factors and treatment with HU.
Supremacy of this study compared to the previous studies is the larger sample size and the ability to compare the presence of silent brain lesions in subgroup of β-TI patients in relation to splenectomy, thrombocytosis, regular blood transfusions, and HU consumption.
In univariate analysis, only splenectomy and thrombocytosis showed statistically significant association with the presence of WML regardless of size and number of the lesions. However, when we stratified patients based on splenectomy status, thrombocytosis showed no significant association with the occurrence of WML in patients with β-TI. It seems that in splenectomized patients additional factors besides thrombocytosis determine the risk of thromboembolic phenomena. Such specific consequences of splenectomy may include the number of circulating pathological RBC which induce thrombin generation [21].
Regular blood transfusions have been reported to prevent the development of stroke in patients with sickle cell anemia due to a decrease in the number of circulating sickle cells [22, 23]. Likewise, in the present study, normal MRI has been found with a higher rate in the patients who were regularly transfused although the correlation was not statistically significant (P = 0.092). Taher et al. [19] have also reported that transfusion naivety could increase the susceptibility of patients with β-TI to develop a higher incidence of brain ischemia. The fact that in the present study this was not the case can be related to the treatment with HU, since in the subgroup of transfusion-independent patients approximately 90 % were treated with HU, and a statistically significant relationship between frequency of WML and HU administration has been found. A suggested mechanism for this effect of HU is significant increase in HbF concomitant with improvement in RBC indices and morphology. The protective effect of HU on the development of stroke in sickle cell disease has been documented [24, 25] since HU increase the percentage of HbF, as well as an increase in Hb levels due to decreasing the imbalance of α/β globin chain synthesis with the consequent improvement in the pathology of the RBC [26]. Moreover, HU can decrease RBC adhesion resulting in decreased vaso-occlusions [27]. Another factor that may decrease the risk of thrombosis is induction of mobilization of arachidonic acid from inner cell membrane aminophospholipids induced by HU in patients with sickle cell anemia [28] as well as decrease of the release of endotheline-I (ET-I), a vasoconstrictor peptide resulting from down regulation of ET-I gene expression [29].
The severity of anemia is another factor that was reported by Manfre et al. [17] and Metarrugcheep et al. [18] to increase the incidence of abnormal MRI findings in patients with β-TI. However, the present results as well as the results of Taher et al. [19] did not confirm this correlation and more studies are warranted to address this point.
One must bear in mind that these results were obtained from cross-sectional studies, which are less significant compared with the longitudinal and interventional studies to determine a precise cause and effect relationship. Therefore, in this study we could only document the presence of possible association between the occurrences of asymptomatic WMLs and the various evaluated factors.
Asymptomatic white matter lesions have been reported to be around 0–11 % in normal individuals less than 50 years old and a higher frequency in older normal individuals (>70 years) possibly due to the impact of age on the pathogenesis of these abnormal changes [30, 31]. The results of the present study compared to normal population in the same age group confirm the increased susceptibility of patients with β-TI to develop asymptomatic cerebral thromboembolism and therefore should be examined on a regular periodic basis, at least once every 2 years in patients >20 years.
Moreover, one should consider preventive anticoagulant and/or antiplatelet aggregates to these patients and possibly designing controlled clinical trial to confirm this assumption. We evaluated a larger cohort of patients with β-TI compared to the previous reports. In univariate analysis both splenectomy and thrombocytosis showed significant correlation with the presence of single or multiple WMLs. However, after adjustment for splenectomy, thrombocytosis showed no significant association with developing asymptomatic brain lesions. Larger studies with prospective design are suggested for better evaluation of the proper effect of associated variables.
References
Eldor A, Rachmilewitz EA (2002) The hypercoagulable state in thalassemia. Blood 99:36–43
Karimi M, Khanlari M, Rachmilewitz EA (2008) Cerebrovascular accident in beta-thalassemia major (beta-TM) and beta-thalassemia intermedia (beta-TI). Am J Hematol 83:77–79
Karimi M, Haghpanah S, Farhadi A et al (2012) Genotype-phenotype relationship of patients with beta-thalassemia taking hydroxyurea: a 13-year experience in Iran. Int J Hematol 95:51–56
Cappellini MD, Robbiolo L, Bottasso BM et al (2000) Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol 111:467–473
Eldor A, Maclouf J, Lellouche F et al (1993) A chronic hypercoagulable state and life-long platelet activation in beta thalassemia major. Southeast Asian J Trop Med Public Health 24(Suppl 1):92–95
Goldschmidt N, Spectre G, Brill A et al (2008) Increased platelet adhesion under flow conditions is induced by both thalassemic platelets and red blood cells. Thromb Haemost 100:864–870
Moratelli S, De Sanctis V, Gemmati D et al (1998) Thrombotic risk in thalassemic patients. J Pediatr Endocrinol Metab 11(Suppl 3):915–921
Naithani R, Chandra J, Narayan S et al (2006) Thalassemia major—on the verge of bleeding or thrombosis? Hematology 11:57–61
Shirahata A, Funahara Y, Opartkiattikul N et al (1992) Protein C and protein S deficiency in thalassemic patients. Southeast Asian J Trop Med Public Health 23(Suppl 2):65–73
Visudhiphan S, Ketsa-Ard K, Tumliang S et al (1994) Significance of blood coagulation and platelet profiles in relation to pulmonary thrombosis in beta-thalassemia/Hb E. Southeast Asian J Trop Med Public Health 25:449–456
Karami H, Vahidshahi K, Kosarian M et al. Assessment of coagulation state and its related factors in thalassemia intermedia patients referred to thalassemia research center at Booali Sina Hospital Sari/IR Iran in 2007. Pak J Biol Sci 13
Musumeci S, Leonardi S, Di Dio R et al (1987) Protein C and antithrombin III in polytransfused thalassemic patients. Acta Haematol 77:30–33
Borenstain-Ben Yashar V, Barenholz Y, Hy-Am E et al (1993) Phosphatidylserine in the outer leaflet of red blood cells from beta-thalassemia patients may explain the chronic hypercoagulable state and thrombotic episodes. Am J Hematol 44:63–65
Shenkman B (2008) Thrombotic complications in thalassemic patients: contribution of red blood cells and platelets. Thromb Haemost 100:735
Taher A, Isma’eel H, Mehio G et al (2006) Prevalence of thromboembolic events among 8,860 patients with thalassaemia major and intermedia in the Mediterranean area and Iran. Thromb Haemost 96:488–491
Karimi M, Bagheri H, Rastgu F et al (2010) Magnetic resonance imaging to determine the incidence of brain ischaemia in patients with beta-thalassaemia intermedia. Thromb Haemost 103:989–993
Manfre L, Giarratano E, Maggio A et al (1999) MR imaging of the brain: findings in asymptomatic patients with thalassemia intermedia and sickle cell-thalassemia disease. AJR Am J Roentgenol 173:1477–1480
Metarugcheep P, Chanyawattiwongs S, Srisubat K et al (2008) Clinical silent cerebral infarct (SCI) in patients with thalassemia diseases assessed by magnetic resonance imaging (MRI). J Med Assoc Thail 91:889–894
Taher AT, Musallam KM, Nasreddine W et al (2010) Asymptomatic brain magnetic resonance imaging abnormalities in splenectomized adults with thalassemia intermedia. J Thromb Haemost 8:54–59
Mikulis DJ, Roberts TP (2007) Neuro MR: protocols. J Magn Reson Imaging 26:838–847
Cappellini MD, Grespi E, Cassinerio E et al (2005) Coagulation and splenectomy: an overview. Ann N Y Acad Sci 1054:317–324
Adams RJ (2000) Lessons from the stroke prevention trial in sickle cell anemia (STOP) study. J Child Neurol 15:344
Adams RJ, McKie VC, Hsu L et al (1998) Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 339:5–11
Ware RE, Zimmerman SA, Sylvestre PB et al (2004) Prevention of secondary stroke and resolution of transfusional iron overload in children with sickle cell anemia using hydroxyurea and phlebotomy. J Pediatr 145:346–352
Gulbis B, Haberman D, Dufour D et al (2005) Hydroxyurea for sickle cell disease in children and for prevention of cerebrovascular events: the Belgian experience. Blood 105:2685–2690
Karimi M, Darzi H, Yavarian M (2005) Hematologic and clinical responses of thalassemia intermedia patients to hydroxyurea during 6 years of therapy in Iran. J Pediatr Hematol Oncol 27:380–385
Johnson C, Telen MJ (2008) Adhesion molecules and hydroxyurea in the pathophysiology of sickle cell disease. Haematologica 93:481–485
AA D, Ghebremeskel K, MI E et al (2011) Hydroxyurea therapy mobilises arachidonic acid from inner cell membrane aminophospholipids in patients with homozygous sickle cell disease. J Lipids 2011
Brun M, Bourdoulous S, Couraud P et al (2003) Hydroxyurea downregulates endothelin-1 gene expression and upregulates ICAM-1 gene expression in cultured human endothelial cells. Pharmacogenomics J 3:215–226
Enzinger C, Smith S, Fazekas F et al (2006) Lesion probability maps of white matter hyperintensities in elderly individuals: results of the Austrian stroke prevention study. J Neurol 253:1064–1070
Longstreth WT Jr, Manolio TA, Arnold A et al (1996) Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 27:1274–1282
Acknowledgments
We would like to thank Shiraz University of Medical sciences for financial and approval support and MRI technicians of Faghihi Hospital Shiraz, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karimi, M., Haghpanah, S., Bagheri, M.H. et al. Frequency and distribution of asymptomatic brain lesions in patients with β-thalassemia intermedia. Ann Hematol 91, 1833–1838 (2012). https://doi.org/10.1007/s00277-012-1527-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-012-1527-x