Abstract
Indoleamine 2,3-dioxygenase (IDO) expression in dendritic cells (DCs) can induce or maintain peripheral immune tolerance. Impaired IDO-mediated tryptophan catabolism has been observed in autoimmune diseases. In order to investigate the effects of IDO-mediated tryptophan catabolism and IDO-expressing DCs in immune thrombocytopenia, the concentrations of kynurenine were detected by high-pressure liquid chromatography. The expressions of IDO were analyzed by flow cytometry and western blot analysis. The effects of IDO+ DCs stimulated with CTLA-4-Ig on T cells proliferation and activation, lymphocyte apoptosis, and Tregs were measured by flow cytometry. We found that the expression of IDO in DCs of immune thrombocytopenia (ITP) patients was significantly decreased. CTLA-4-Ig significantly increased the expression of functional IDO in DCs of ITP patients. IDO+ DCs stimulated with CTLA-4-Ig suppressed T cells proliferation and activation, promoted lymphocyte apoptosis, and increased the percentage of Tregs. These results suggest that decreased IDO expression in DCs may play a critical role in ITP. CTLA-4-Ig successfully corrected the disorder of IDO expression in ITP. IDO+ DCs stimulated with CTLA-4-Ig inhibited immune responses by an IDO-dependent mechanism. Increasing the expression and activity of IDO in DCs might be a promising therapeutic approach for ITP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune thrombocytopenia (ITP) is a common autoimmune disease characterized with a low platelet count and mucocutaneous bleedings. In ITP, the immune tolerance to platelet autoantigens is impaired, resulting in immune-mediated platelet destruction and/or suppression of platelet production [1]. However, the mechanism(s) governing the breakdown of immune tolerance to platelet autoantigens that leads to autoantibody production remains obscure.
Indoleamine 2,3-dioxygenase (IDO) is the first enzyme which also catalyzes the rate-limiting step in the metabolism of tryptophan. Activation of this pathway reduces the bioavailability of tryptophan to T cells and generates the proapoptotic tryptophan metabolite, kynurenine. Together, this leads to apoptosis and inhibition of T cells proliferation [2, 3]. Tryptophan is an essential amino acid for cell survival and proliferation. The depletion of tryptophan can arrest proliferating cells in mid-G1 phase and block the effector activity of T cells [4, 5]. The accumulation of proapoptotic tryptophan-derived metabolites, such as kynurenine, is toxic for T cells, B cells, and NK cells and induces cell death by apoptosis [3, 4]. IDO is a key immunomodulatory enzyme that can induce or maintain peripheral immune tolerance and immunosuppression. Recently, impaired IDO-mediated tryptophan catabolism has been observed in many patients with autoimmune diseases such as multiple sclerosis, autoimmune diabetes, Graves' disease, and rheumatoid arthritis [6–9].
Dendritic cells (DCs) are critical in initiating and regulating immune responses and can play either suppressive or stimulatory roles, depending on whether prevailing conditions lead to induction of biologically active IDO [10]. Regulatory DCs expressing high levels of active IDO are important in the generation and maintenance of peripheral tolerance via depletion of autoreactive T cells and induction of regulatory T cells (Tregs) responses [11]. However, whether disregulated expression and enzymatic activity of IDO in DCs plays a pathogenic role in ITP remains unknown.
Materials and methods
Patients and controls
Fifteen patients diagnosed with ITP (nine females and six males, age range 17–64 years, median 38 years; platelet counts ranged from 3–36 × 109/L, with a median count of 12 × 109/L) were selected for this study (Supplementary Table 1). Of these patients, five with newly diagnosed and ten with recurrent ITP (platelet counts <50 × 109/L) had not been treated for at least 1 month prior to the sampling. Patients complicated with Graves' disease or connective tissue diseases, such as systemic lupus erythematosus, were excluded. The normal control group consisted of 12 healthy adult volunteers (7 females and 5 males, age range 21–59 years, median 39 years). Platelet counts ranged from 126–287 × 109/L, with a median count of 197 × 109/L. Enrollment took place between January 2009 and October 2010, at the Department of Hematology, Qilu Hospital, Shandong University. All of the cases met the diagnosis criteria of ITP as previously described [12]. Informed consent was obtained from each patient and healthy control in accordance with the Declaration of Helsinki.
Preparation of platelets and cells
Heparinized peripheral blood was centrifuged at 200 × g for 15 min at 20 °C. Autologous platelets were separated from the platelet-rich plasma. PBMCs were isolated from the substratum cell layer using 1.077 g/mL Ficoll-Hypaque (Invitrogen, Carlsbad, CA) gradient centrifugation (800 × g for 20 min, 20 °C). The isolated PBMCs and autologous platelets were washed twice with 0.9 % NaCl and then resuspended at 1 × 106 PBMCs/mL and 1 × 107 platelets/mL for cell culture.
DCs preparation and determination of the expression of IDO by flow cytometry
DCs were generated according to a protocol as previously described [13]. PBMCs were incubated at 37 °C and 5 % CO2 for 4–6 h, and nonadherent cells were removed. The adherent cells were cultured for 7 days in a serum-free RPMI 1640 medium with 50 ng/mL GM-CSF (R&D Systems, Minneapolis, MN, USA) and 50 ng/mL IL-4 (R&D Systems), added on days 0, 2, and 4. Optimal conditions were maintained by replacing half of the media with a new medium containing fresh cytokines. The nonadherent fraction from the culture was harvested by gentle aspiration and treated for 48 h with the following mixture: LPS (250 ng/mL; Sigma-Aldrich, USA), IL-1β (2 ng/mL; R&D Systems), IL-6 (100 ng/mL; R&D Systems), and PGE2 (1 μg/mL; Alexis-Biochemicals, San Diego, CA) [14]. The nonadherent fraction (DCs) was harvested by gentle aspiration. The cell surface markers and the expression of IDOl were analyzed by flow cytometry. The result showed that IDO+ cells constituted up to 90 % of these cells. This was determined by staining, after fixation and permeabilization, with purified rabbit antihuman IDO mAb (2 μg; R&D Systems) for 30 min and subsequently with phycoerythrin (PE)-Cy5.5-labeled goat anti-rabbit IgG (0.2 μg; Invitrogen) for 15 min. This anti-IDO antibody is specific for IDO1. In order to determine the phenotypic characterization of IDO+ DCs, IDO+ DCs (1 × 106) in a total volume of 100 μL were incubated with 20 μL fluorescein isothiocyanate (FITC)-conjugated LIN1 (BD Biosciences, San Jose, CA), FITC-conjugated CD1a, PE-conjugated CD11c, PE-conjugated CD123, PE-Cy5-conjugated HLA-DR, FITC-conjugated CD83, or PE-conjugated CD86 (BioLegend, San Diego, CA) at 4 °C for 30 min and analyzed within 1 h by flow cytometry.
Effects of CTLA-4-Ig on the expression and activity of IDO in DCs of ITP patients
DCs were cultured in the presence of CTLA-4-Ig (CTLA-4-Ig 10 μg/mL, group 1) or absence of CTLA-4-Ig (CTLA-4-Ig 0 μg/mL, group 2) for 2 days. The cells were harvested. The expression of IDOl was analyzed by flow cytometry (idem), real-time reverse transcription (RT)-PCR, and western blot analysis.
Real-time RT-PCR
For reverse transcription-polymerase chain reaction, TRIzol (1 mL; Invitrogen) was used to isolate the total RNA. RNA was converted into cDNA using the PrimeScript™ RT reagent kit (Perfect Real Time; Takara Biotechnology, Inc., Japan) according to the manufacturer's instructions. The primers for IDO1 and the endogenous control (GAPDH) are as follows: IDO1 forward, 5′-CGGTCTGGTGTATGAAGGGT-3′; IDO1 reverse, 5′-TGGCTTGCAGGAATCAGGAT-3′; GAPDH forward, 5′-GTGAAGGTCGGAGGAGTCAACG-3′; and GAPDH reverse, 5′-TGAGGTCAATGAAGGGGTC-3′. Multiplex RT-PCR for IDO and GAPDH was performed on a LightCycler® 2.0 (Roche Applied Science, Switzerland) using SYBR Green (Takara Biotechnology) as a double-strand DNA-specific binding dye. The primers for all mRNA assays were intron spanning. The PCR reactions were cycled 40 times after initial denaturation (95 °C, 5 min) with the following parameters: denaturation at 95 °C for 15 s, annealing at 55 °C for 15 s, and extension at 72 °C for 35 s, with temperature transition rates of 20 °C/s.
Samples were analyzed using comparative Ct method (using arithmetic formula) for relative quantification of IDO mRNA according to the LightCycler software version 4 (Roche) [15]. The amplification efficiency between the target (IDO) and the reference control (GAPDH) was compared in order to use the ΔΔCt calculation.
Western blot analysis
DCs (2.5 × 106) were pelleted by centrifugation and resuspended in lysis buffer. Equal amounts of protein (20 μg) were loaded onto a 5 % acrylamide stacking gel and separated by SDS-PAGE using a 10 % separating gel. Following transfer of separated proteins, nitrocellulose membranes were blocked and probed overnight at 4 °C with rabbit antihuman IDO mAb (1 μg/mL; R&D Systems). The membrane was then probed for 1 h at room temperature with goat anti-rabbit peroxidase-conjugated IgG (1 μg/mL; Jingmei-Biotech, China), and the immunoreactivity was detected by chemiluminescence. To quantify IDO proteins, each band density was normalized to β-actin protein.
High-pressure liquid chromatography
Kynurenine levels were measured by high-pressure liquid chromatography (HPLC) to determine IDO enzyme activity. DCs were cultured in the presence CTLA-4-Ig (CTLA-4-Ig 10 μg/mL, group 1) with or without D-1MT (200 μM; Sigma-Aldrich) or absence of CTLA-4-Ig (CTLA-4-Ig 0 μg/mL, group 2) for 48 h. All groups were cultured with exogenous l-tryptophan (150 μM; Sigma-Aldrich). After culturing, the supernatant was collected, and its kynurenine concentration was measured. In brief, 400 μL of supernatant of culture was diluted with 400 uL of potassium phosphate buffer (0.05 M, pH 6.0), and the protein was precipitated with 100 μL of 2 M trichloroacetic acid. Supernatants (300 uL) were then injected into an RP18 column and eluted with a degassed potassium phosphate solution (0.015 M; pH 6.4) containing 27 mL/L acetonitrile at a flow rate of 0.5 mL/min. Kynurenine was detected using a UV detector at a wavelength of 350 nm. The values were referred to a standard curve with defined kynurenine concentrations (0–60 μM).
Platelet membrane glycoprotein-reactive T cell cultivation
PBMCs (106/well) and autologous platelets (107/well) were cocultured in an RPMI 1640 medium supplemented with 10 % fetal calf serum (FCS) in a 24-well culture plate (1 mL final volume; BD Biosciences) at 37 °C with 5 % CO2, with PHA (1.0 μg/mL; Sigma-Aldrich), IL-2 (50 U/mL; Peprotech, New Jersey, USA), and autologous antigen-presenting cells (APCs) exposed to 3,300 rad60Co. Optimal conditions were maintained by replacing half of the media every 2–3 days with a new medium containing fresh cytokines, autologous irradiated APCs, and autologous platelets. Platelets glycoprotein (GP)-specific T cells were harvested after 7 days of coculture [16].
Mixed lymphocyte reactions
Mixed lymphocyte reaction (MLR)-1 was performed by addition of IDO+ DCs (105/well), autologous platelets, and autologous platelet GP-specific T cells (106/well) in an RPMI 1640 medium supplemented with 10 % FCS without addition of tryptophan in a 24-well culture plate at 37 °C with 5 % CO2, with IL-2 (50 U/mL; Peprotech, New Jersey, USA) for 2 days. Cultures were set up in the presence or absence of the IDO inhibitor D-1MT (200 μM; Sigma-Aldrich).
MLR-2 was performed by addition of IDO+ DCs (105/well), autologous platelets (107/well), and PBMCs (106/well) in an RPMI 1640 medium supplemented with 10 % FCS in 24-well culture plates at 37 °C with 5 % CO2, with IL-2 (50 U/mL; Peprotech, New Jersey, USA) for 48 h.
Effects of IDO-expressing DCs stimulated by CTLA-4-Ig on proliferation, activation, apoptosis of lymphocytes, and Tregs by flow cytometry
T cell proliferation
Cells (1 × 105) from the MLR-1 coculture were transferred to a 96-well U-bottom culture plate. Proliferation was assessed using Quick Cell Proliferation Assay Kit (BioVision, Mountain View, CA) according to the manufacturer's protocol.
T cell activation
CD3+ T cells were obtained using streptavidin-conjugated immunomagnetic beads (50 μL; Jingmei Biotech) binding to mouse antihuman CD3/biotin-labeled mAb (1.0 μg for 1 × 106 cells; Jingmei Biotech) and MLR-2-cocultured T cells (106) in a total volume of 100 μL. These CD3+ T cells were incubated with 20 μL PE-labeled CD69 (eBioscience, San Diego, CA), PE-Cy5-labeled CD71 (BD Biosciences), and APC-labeled CD25 (eBioscience) for 30 min at 4 °C and analyzed by flow cytometry.
Determination of the apoptosis of lymphocytes
Cells (106) from MLR-2 cocultures were harvested and incubated with 20 μL PE-Cy5-conjugated CD1, PE-conjugated CD8, or PE-Cy5-conjugated CD4 (BD Biosciences, San Jose, CA, USA) for 30 min. Cells were washed and incubated with 5 μL FITC-conjugated annexin V (Invitrogen) for 15 min and analyzed within 1 h by flow cytometry.
Determination of Tregs
CD4+CD25+ Foxp3+ (Tregs) cells were assessed using a human regulatory T cell staining kit (with PE Foxp3 PCH101, FITC CD4, APC CD25; Treg kit; eBioscience). In brief, cells (106) from MLR-2 cocultures were incubated with 20 μL CD4/25 cocktail (1 μg CD4 and 0.125 μg CD25) in a total volume of 100 μL for 30 min at 4 °C. Cells were washed with 2 mL cold PBS and incubated with 1 mL freshly prepared fixation/permeabilization buffer for 45 min. After washing with 2 mL 1× permeabilization buffer, 20 μL antihuman Foxp3 antibody was added and incubated for 30 min. Cells were washed twice and analyzed by flow cytometry.
Statistical analysis
Data were expressed as mean ± SD. Statistical significance was determined by ANOVA. All tests were performed by SPSS 13.0 system. A P value less than 0.05 was considered to be statistically significant.
Results
Expression of IDO in DCs of ITP patients
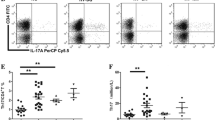
In order to further study the expression of IDO in DCs of ITP patients, the monocyte-derived DCs of ITP patients and normal human were induced and generated with cytokines (GM-CSF, IL-4, LPS, IL-1β, IL-6, and PGE2) in vitro. Compared with that in normal controls, the mean fluorescence intensity (MFI) of IDO had a significant decrease in DCs of ITP patients (376.9 ± 28.00 vs 124 ± 23.10, P < 0.01) (Fig. 1).
The effects of CTLA-4-Ig on the expression and activity of IDO in DCs of ITP patients
The DCs were CD1a+, CD11c+, CD123−, CD83+, and expressed high levels of HLA-DR and CD86, consistent with a mature DC phenotype (data not shown). In order to further study the effects of CTLA-4-Ig on the expression and activity of IDO in DCs of ITP patients, DCs were cultured in the presence of CTLA-4-Ig (group 1) or absence of CTLA-4-Ig (group 2). The IDO expression was detected by real-time RT-PCR, western blot, and flow cytometry. We found that IDO expression in group 1 was significantly higher than that in group 2. The relative amount of IDO mRNA in group 1 was 36.8-fold (P = 0.0004) greater than that in group 2 (Fig. 2a). This was confirmed by western blot analysis (Fig. 2b) and flow cytometric analysis, in which the MFI of IDO was significantly higher in DCs of group 1 than that of group 2 (401.0 ± 44.9 vs 124.0 ± 23.1; P < 0.0001) (Fig. 2c).
The effects of CTLA-4-Ig on the expression and activity of IDO in DCs. (a) IDO mRNA expression in group 1 (CTLA-4-Ig 10 μg/mL) was significantly higher than that in group 2 (CTLA-4-Ig 0 μg/mL) (P = 0.0004). The result suggested that CTLA-4-Ig significantly increased the IDO mRNA expression in DCs of ITP patients. (b) The IDO MFI was significantly increased in DCs of group 1 compared with that in DCs of group 2 (401.0 vs 124.0; P < 0.0001). (c) The western blot analysis showed IDO expression in DCs of group 1 was significantly higher than that in DCs of group 2. The result further confirmed that CTLA-4-Ig significantly improved the IDO expression in DCs of ITP patients. (d) The kynurenine concentration of supernatant was significantly higher in group 1 than that in group 2 (0.86 vs 0.39 uM; P = 0.0179). However, after adding the IDO-specific inhibitor D-1MT to block the IDO activity in group 1, the kynurenine concentration in the supernatant significantly decreased (0.86 vs 0.19 uM; P = 0.0108). These findings suggested that CTLA-4-Ig significantly enhanced the expression of enzymatically active IDO in DCs from ITP patients. Bars represent SD. *P < 0.05; **P < 0.01
In order to evaluate the activity of IDO, we measured the kynurenine concentrations from culture supernatant with HPLC and found that kynurenine was significantly higher in group 1 than in group 2 (0.86 ± 0.08 vs 0.39 ± 0.03 uM; P = 0.0179). However, after adding the IDO-specific inhibitor D-1MT to block IDO activity in group 1, the kynurenine concentration in the supernatant significantly decreased (0.86 ± 0.08 vs 0.19 ± 0.05 uM; P = 0.0108) (Fig. 2d).
The effects of IDO-expressing DCs stimulated with CTLA-4-Ig on proliferation, activation, apoptosis of lymphocytes, and Tregs
Using a MLR coculture assay, we assessed the effects of IDO+ DCs stimulated with CTLA-4-Ig on T cell proliferation and activation. In the absence of D-1MT, IDO+ DCs from ITP patients, which are stimulated with CTLA-4-Ig, significantly inhibited autologous platelet GP-reactive T cells proliferation (0.43 ± 0.10 vs 0.65 ± 0.06; P < 0.0001), while in the absence of CTLA-4-Ig, they could not. However, when the IDO activity was blocked with D-1MT, IDO+ DCs were capable of strongly driving autologous platelet GP-reactive T cells responses (0.74 ± 0.04 vs 0.43 ± 0.10; P < 0.0001) (Fig. 3a), indicating that this culture condition (in the presence/absence of D-1MT) caused DCs to lose their suppressive capacity and, instead, play a stimulatory role.
The effects of CTLA-4-Ig on proliferation, activation, apoptosis of lymphocytes, and Tregs. (a) IDO+ DCs from ITP patients stimulated with CTLA-4-Ig significantly inhibited the autologous platelet GP-reactive T cells proliferation (0.43 vs 0.65; P < 0.0001), but, when IDO activity was blocked with 1-MT, IDO+ DCs were capable of strongly driving the autologous platelet GP-specific T cells responses (0.74 vs 0.43; P < 0.0001). (b) The T cell activation was significantly inhibited by IDO+ DCs induced by CTLA-4-Ig. Compared with group 2 (CTLA-4-Ig 0 μg/mL), the percentage of CD25+, CD69+, and CD71+ cells significantly decreased in group 1 (CTLA-4-Ig 10 μg/mL). (c) IDO+ DCs induced by CTLA-4-Ig significantly increased the apoptosis of lymphocytes. The annexin V percentage of CD4+, CD8+, and CD19+ cells significantly increased in group 1. (d) The percentage of CD4+CD25+ Foxp3+ (Tregs) cells in group 1 was significantly higher than that in group 2 (5.8 vs 2.7 %; P = 0.0001). (e) CD4+CD25+ Foxp3+ (Tregs) cells were assessed by flow cytometry. This figure show representative flow cytometric dot plots in different groups. Bars represent SD. *P < 0.05; **P < 0.01
The surface expression of the T cell activation markers CD25, CD69, and CD71 on T cells was assessed. Compared with group 2 (without CTLA-4-Ig), the T cell activation was significantly inhibited in group 1. The percentage of CD3+/CD25+, CD3+/CD69+, and CD3+/CD71+ cells in group 1 was 22.7 ± 4.1, 8.0 ± 6.5, and 24.4 ± 10.4 %, respectively, and, in group 2, was 31.0 ± 4.7, 15.0 ± 11.0, and 32.0 ± 12.5 %, respectively, (Fig. 3b).
The effect of IDO+ DCs stimulated with CTLA-4-Ig on lymphocyte apoptosis was also analyzed by flow cytometry. Compared with group 2, the apoptosis of lymphocytes was significantly increased in group 1. The annexin V percent on CD4+, CD8+, and CD19+ cells in group 1 was 21.8 ± 7.8, 10.7 ± 3.1, and 18.9 ± 9.9 %, respectively, and, in group 2, was 13.8 ± 6.1, 7.8 ± 2.9, and 13.3 ± 9.3 %, respectively, (Fig. 3c).
IDO+ DCs stimulated with CTLA-4-Ig (group 1) also significantly increased the percentage of CD4+CD25+ Foxp3+ (Tregs) cells. The percentage of Tregs in group 1 was significantly higher than that in group 2 (5.8 ± 1.7 vs 2.7 ± 0.9 %; P = 0.0001) (Fig. 3d). Figure 3e represents the percentage of Tregs in different groups in a typical ITP patient measured by flow cytometry.
Discussion
As an important regulator of immune balance in physiological conditions [17, 18], IDO can promote peripheral immune tolerance and control autoimmune responses through tryptophan catabolism. Impaired IDO-mediated tryptophan catabolism has been demonstrated in some autoimmune diseases. In this study, we found that the expression of IDO was impaired in DCs of ITP patients. We isolated and cultured monocyte-derived DCs of ITP patients and normal human in vitro and found that the expression of IDO in DCs of ITP patients was significantly decreased compared with that of normal controls. However, some studies showed that ITP patients had significant increase of IDO expressions in PBMCs, but the activity of IDO was insufficient [19]. It is possible that the varieties and levels of inflammatory cytokine were varied in vitro and in vivo. IDO is transcriptionally induced by a variety of inflammatory cytokine such as interferon-γ (IFN-γ), IFN-α, and tumor necrosis factor alpha [20]. ITP is an autoimmune disorder manifested by an imbalance of the Th1/Th2 ratio [21]. The interferon-γ level is significantly increased in ITP patients. Elevated IFN-γ can upregulate the expression of IDO. Therefore, the expression of IDO of ITP patients is significantly higher than that of normal controls whose interferon-γ level is normal in vivo. However, in vitro, in the same cultivation condition, the expression of IDO significantly decreased in DCs of ITP patients compared with that of normal controls, which meant that the expression of IDO was defective in DCs of ITP patients.
DCs play crucial roles in the initiation and regulation of immune responses. IDO upregulation in DCs can inhibit T cell activation and proliferation and induce T cells apoptosis [22]. IDO+ DCs can suppress Ag-specific T cells responses in vitro [23]. ITP is an acquired autoimmune disease, which is mediated by autoreactive B cell and T cell clones. CD4+ T cells autoreactive to platelet gpIIb-IIIa have been identified in patients with ITP [24], but the trigger for T cell dysregulation in ITP is unclear. In our study, we found that the expression of IDO in DCs of ITP patients was significantly decreased. The decreased expression of IDO in DCs could reduce the depletion of available tryptophan and the generation of cytotoxic tryptophan catabolites, such as kynurenine, and thereby leads to the increasing survival of autoreactive T cells in ITP.
It was well known that IDO is strongly expressed in macrophages and DCs [13], and its enzymatic activity is strictly regulated [25]. The triggering of functional IDO requires the ligation of B7-1/B7-2 molecules on the DCs by CTLA-4/CD28, especially CTLA-4 [11]. CTLA-4 is a negative regulator of T cells activation. Data from previous studies indicated that CTLA-4 on T cells or soluble CTLA-4-Ig can increase IDO expression and activate IDO enzyme in DCs by binding to and inducing signaling through B7 molecules [26]. In our study, we found that CTLA-4-Ig significantly increased the expression and activity of IDO in DCs of ITP. Furthermore, we evaluated the functional consequences of IDO activity exerted by those IDO+ DCs. We found that IDO+ DCs induced in the presence of CTLA-4-Ig significantly suppressed the proliferation of autologous platelet GP-reactive T cells in ITP. In addition, we also found that IDO+ DCs induced in the presence of CTLA-4-Ig remarkably suppressed the activation of T cells and promoted the apoptosis of CD19+ and CD8+, especially CD4+ cells. D-1MT, a specific inhibitor of IDO, blocked the effect of IDO+ DCs induced by CTLA-4-Ig on the proliferation of autologous platelet GP-reactive T cells, indicating that the effect was IDO-dependent.
CD4+CD25+ regulatory T cells (Tregs) play a critical role in the maintenance of peripheral immune tolerance. Decreased number and function of Treg cells have been found in ITP [27]. It was well known that regulatory DCs expressing high levels of active IDO can induce and maintain peripheral tolerance by depletion of autoreactive T cells and induction of regulatory T cells. But, in ITP, whether the reduced expression of IDO in DCs leading to the abnormality of Treg cells remains unknown. Data from our study demonstrated that IDO+ DCs induced with CTLA-4-Ig significantly increased the percentage of CD4+CD25+ Foxp3+ (Tregs) cells in ITP. Several lines of evidence suggest that IDO-positive DCs can induce or activate Tregs. IDO activity in mouse splenic DCs supports the conversion of CD4+CD25− T cells into Foxp3-expressing Tregs [28]. In addition, a subset of IDO-expressing pDCs in mouse tumor-draining lymph nodes directly activates resting Tregs [17]. Tregs can suppress T cells activation and induce immune tolerance, which provides an additional level of immune regulation beyond the tryptophan deprivation and generation of proapoptotic tryptophan metabolites. Tryptophan is an essential amino acid that is required by all cells to synthesize proteins. Whether Tregs have a survival advantage over the other autoreactive T cells, due to decreased susceptibility to tryptophan deprivation and kynurenine catabolite toxicity, requires further investigation.
In summary, the expression of IDO in DCs of ITP patients was significantly decreased. CTLA-4-Ig successfully enhanced the expression of functional IDO in DCs of ITP patients. IDO+ DCs stimulated with CTLA-4-Ig suppressed the proliferation and activation of T cells, promoted the apoptosis of lymphocytes, and increased the percentage of Tregs by an IDO-dependent mechanism. These results indicated that decreased IDO expression in DCs may play a critical role in ITP. Increasing the expression of functional IDO in DCs might be a promising therapeutic approach for ITP.
References
McMillan R (2007) The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol 44(4 suppl 5):S3–S11
Shimizu T, Nomiyama S, Hirata F, Hayaishi O (1978) Indoleamine 2,3-dioxygenase: purification and some properties. J Biol Chem 253(13):4700–4706
Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P (2002) T cell apoptosis by tryptophan catabolism. Cell Death Differ 9(10):1069–1077
Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB (2002) Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 196(4):459–468
Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL (2005) GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22(5):633–642
Zhu L, Ji F, Wang Y, Liu Q, Zhang JZ, Matsushima K, Cao Q, Zhang Y (2006) Synovial autoreactive T cells in rheumatoid arthritis resist IDO-mediated inhibition. J Immunol 177(11):8226–8233
Munn DH, Mellor AL, Rossi M, Young JW (2005) Dendritic cells have the option to express IDO-mediated suppression or not. Blood 105(6):2618
Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E (2004) Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest 114(2):270–279
Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, Samelson LE, Thompson CB, Bluestone JA (1998) Molecular basis of T cell inactivation by CTLA-4. Science 282(5397):2263–2266
Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC et al (2002) CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol 3(11):1097–1101
Munn DH, Sharma MD, Mellor AL (2004) Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol 172:4100–4110
Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N et al (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113(11):2386–2393
Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R et al (2002) Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 297(5588):1867–1870
Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH (1997) Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum free conditions. Eur J Immunol 27(12):3135–3142
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):e36
Ware RE, Howard TA (1993) Phenotypic and clonal analysis of T lymphocytes in childhood immune thrombocytopenic purpura. Blood 82(7):2137–2142
Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH (2007) Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 117(9):2570–2582
Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N et al (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113(11):232386–232393
Wang CY, Shi Y, Min YN, Zhu XJ, Guo CS, Peng J, Dong XY, Qin P, Sun JZ, Hou M (2011) Decreased IDO activity and increased TTS expression break immune tolerance in patients with immune thrombocytopenia. J Clin Immunol 31(4):643–649
King NJ, Thomas SR (2007) Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol 39(12):2167–2172
Semple JW, Milev Y, Cosgrave D et al (1996) Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood 87(10):4245–4254
Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA (2000) Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol 164(7):3596–399
Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4(10):762–774
Sukati H, Watson HG, Urbaniak SJ, Barker RN (2007) Mapping helper T-cell epitopes on platelet membrane glycoprotein IIIa in chronic autoimmune thrombocytopenic purpura. Blood 109(10):4528–4538
Thomas SR, Terentis AC, Cai H, Takikawa O, Levina A, Lay PA, Freewan M, Stocker R (2007) Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem 282:23778–23787
Mellor AL, Baban B, Chandler P et al (2003) Cutting edge: induced indoleamine 2,3-dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol 171(4):1652–1655
Liu B, Zhao H, Poon MC, Han Z, Gu D, Xu M, Jia H, Yang R, Han ZC (2007) Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol 78(2):139–143
Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C (2006) The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 176(11):6752–6761
Acknowledgments
This work was supported by grants from Tai Shan Scholar Foundation, National Natural Science Foundation of China (numbers 81170475, 30800491, 30801258, 30971278, 81070396, 81070408, 81070407, 81070411, 81100334, 81100335, 81100336, 81100348, 81101869, and 81170515), National Science Fund for Distinguished Young Scholars (number 81125002), 973 Program (2009CB521904, 2011CB503906), Key Project of Chinese Ministry of Education (109097), Key Clinical Research Project of Public Health Ministry of China 2010–2012, Natural Science Foundation of Shandong Province (ZR2009CM001, ZR2010HQ002, ZR2010CQ040), National Key Clinical Specialist Vocational School about clinical speciality for blood disorders, Clinical Medicine Center Foundation of Shandong Province, Leading Medical Professionals Foundation of Shandong Province, Outstanding Young Scientist Research Award Foundation of Shandong Province (2009BSB01001, BS2009SW014, BS2010YY024, BS2010YY039, BS2011SW013, BS2011YY021), the Research Fund for the Doctoral Program of Higher Education of China (20100131120058), and the State Program of National Natural Science Foundation of China for Innovative Research Group 2011–2013 (81021001).
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shu-qian Xu, Chun-yan Wang, and Xiao-juan Zhu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Clinical characteristics of ITP patients. (DOC 40 kb)
Rights and permissions
About this article
Cite this article
Xu, Sq., Wang, Cy., Zhu, Xj. et al. Decreased indoleamine 2,3-dioxygenase expression in dendritic cells and role of indoleamine 2,3-dioxygenase-expressing dendritic cells in immune thrombocytopenia. Ann Hematol 91, 1623–1631 (2012). https://doi.org/10.1007/s00277-012-1451-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-012-1451-0