Abstract
Different RIC regimens were evaluated prior to allo-HSCT in different hematological malignancies. We conducted this prospective study in adult patients with various hematological malignancies in order to evaluate the toxicity and efficacy of treosulfan-based conditioning, followed by allo-HSCT from 10/10 HLA-identical unrelated donors. Conditioning included treosulfan 12 g/m2/day i.v. (day −6 to day −4), fludarabine 30 mg/m2/day i.v. (day −6 to day −2), and ATG 2.5 mg/kg/day (day −2 to day −1). PBSC were used as HSC source. We included 56 patients (29 AML, 9 MM, 8 MDS, 6 CLL, 3 ALL, and 1 CML) with a median age of 57 years (18–65.5). Fifty-four (96%) patients engrafted; the cumulative incidence of aGVHD grade ≥II at 3 months reached 31%. The cumulative incidence of cGVHD at 18 months was 34% limited and 8% extensive. The median overall survival (OS) was not reached with a 3-year probability of 52%. The cumulative incidence of relapse at 3 years was 25%, and the cumulative incidence of transplant-related mortality (TRM) at 12 and 24 months was 20% and 23%, respectively. Treosulfan appears to be a good alternative for conditioning of MUD transplant patients with promising results in terms of OS, relapse, and TRM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) nowadays represents a curative approach for a number of hematological malignancies. The growing number of international volunteer donor registries increased the availability and use of hematopoietic stem cell (HSC) from unrelated donors and allowed to obtain similar results to those after allo-HSCT from related donors [1, 2]. This was especially due to the important development of human leukocyte antigen (HLA) knowledge and typing techniques leading to the improvement in donor selection, and was also due to the better management of transplantation complications [3–5]. The major focus in the allo-HSCT settings was to find the optimal balance between the graft-versus-host disease (GVHD) and the graft-versus-leukemia (GVL) effect, one of the main factors was by adjusting the conditioning intensity. Dose-intensive standard myeloablative conditioning (MAC) regimens followed by allo-HSCT can lead to a lower relapse incidence and prolonged remissions [6–8]. However, these results are associated with an increased risk of transplant-related mortality (TRM) [9, 10] even among young patients and those who have less comorbidities. On this account, different reduced-intensity conditioning (RIC) regimens were developed to overcome high rates of TRM and confer better transplantation outcomes [11, 12], but the main issue was the higher relapse rate after RIC compared to MAC. Treosulfan is a prodrug of a bifunctional alkylating agent possessing both myelotoxic and immunosuppressive properties; it has previously been evaluated in the treatment of solid tumors including ovarian cancer [13]. Treosulfan associated to fludarabine was recently used as conditioning regimen in many clinical studies prior to allo-HSCT for the treatment of different hematological malignancies leading to promising results either in terms of toxicity or efficacy [14–22]. Treosulfan-based conditioning can probably offer a less toxic regimen than MAC with a full intensity and efficacy. Dose range finding and phase II clinical trials revealed treosulfan doses of 3 × 12 to 3 × 14 g/m2 as feasible and effective when combined with fludarabine for additional pre-transplant immunosuppression [14, 23]. This multicenter prospective phase II trial was designed to evaluate the efficacy and toxicity of treosulfan, fludarabine, and anti-thymocyte globulin (ATG) association as conditioning regimen followed by matched unrelated donor (MUD) allo-HSCT in patients with various hematological malignancies in stable disease or in remission, but considered ineligible for MAC. Our main objective was to study the overall survival (OS) at 1 year. Secondary objectives were: engraftment, GVHD incidence, chimerism analysis, event-free survival (EFS), and toxicity.
Design and methods
Criteria for eligibility
This phase II prospective study included adult patients aged 18 years or older presenting hematological malignancies that would gain benefit from allo-HSCT. Accepted diagnoses were: chronic myeloid leukemia (CML) in first chronic phase (CP) resistant to imatinib, in second CP, or in complete remission (CR) after blast crisis; multiple myeloma (MM) or chronic lymphocytic leukemia (CLL) in stage B or C, in partial remission after relapse post-autologous HSCT; acute lymphocytic or myeloid leukemia (ALL, AML) patients in CR1 at high risk or >CR1, and myelodysplastic syndromes (MDS) with poor prognostic factors. Patients with poor performance status (Karnofsky score <70%) or having one of the following criteria: HIV seropositivity, inadequate renal or liver function, severe heart failure, severe concomitant neurological or psychological disease, pregnancy or lactation were not considered as eligible. The trial was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the local ethics committees at the participating institutions. Written informed consent was obtained for all participants.
Donor selection
All unrelated donors had to be 10/10 HLA identical on HLA-A, -B, -C, -DRB1, and -DQB1 on allelic level. Donors were mobilized for peripheral blood stem cells (PBSC) collection using G-CSF 10 μg/kg/day for five consecutive days.
Conditioning and GVHD prophylaxis
Based on previously conducted dose range finding and phase II clinical trials and considering the eligible patient population of this clinical protocol (aged patients with intensive pretreatment and comorbidities), a dose of 3 × 12 g/m2 treosulfan was recommended. In detail, the conditioning included: treosulfan (provided by medac GmbH, Hamburg, Germany) 12 g/m2/day i.v. (day −6 to day −4), fludarabine (Schering GmbH Berlin, Germany) 30 mg/m2/day i.v. (day −6 to day −2), and anti-thymocyte globulin (ATG; Genzyme Inc.) 2.5 mg/kg/day (day −2 to day −1). Anticonvulsive prophylaxis was not administered. GVHD prophylaxis used ciclosporine-A oral (5 mg/kg/day) or i.v. (3 mg/kg/day) from day −1; beyond day 21, doses were adjusted according to disease response and chimerism results. In case of minor ABO incompatibility, methotrexate 15 mg/m2 was administered i.v. at day 1, then 10 mg/m2 at days 3, 6, and 11 [24].
Chimerism analysis
Chimerism analysis was performed on marrow and/or blood samples (total white blood cells and/or CD3+ cells) using polymerase chain reaction based on informative polymorphic short tandem repeat with an accuracy of ±5%, at 1, 2, 3, 4, 6, 9, and 12 months after transplantation.
Adverse event reporting
Adverse events were recorded from the start of the conditioning regimen up to 12 months after transplantation according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE version 3.0).
Endpoint description
Neutrophil recovery was defined by the first day of an absolute neutrophil count ≥500 cells/mm3 for three consecutive days; platelet recovery was defined by the first day of a count ≥50,000 cells/mm3 for seven consecutive days without any transfusion support. GVHD was reported and graded according to published criteria [25]. Chronic GVHD was diagnosed according to standard criteria in patients who survived at least 90 days after transplantation [26]. TRM was defined as death from any cause other than relapse occurring after transplantation. Relapse was defined on the basis of morphologic evidence of hematopoietic disease in bone marrow or other sites. OS was defined as the time from transplantation to any cause of death, and EFS was defined as survival from transplantation to disease progression or death.
Statistical analysis
Cumulative incidence curves were used to estimate incidence over time for neutrophil and platelet recovery, acute and chronic GVHD, TRM, and relapse. OS and EFS were estimated by the Kaplan–Meier method with log-rank test for univariate analysis [27]. Cox proportional hazards regression models were used to assess the influence of disease- and transplant-related variables on OS and EFS [28]. This multivariate analysis studied the following variables: age, cytogenetics, type of disease, number of previous treatments, sex matching, ABO compatibility, cytomegalovirus (CMV) matching, disease status at transplantation, interval diagnosis–allo-HSCT, and CD34+ infused cell number. Statistical analysis was performed with R statistical software (version 2.9.2).
Results
Patients
Between February 2005 and June 2009, 56 patients from five French centers were enrolled, 30 (54%) males and 26 (46%) females with a median age of 57 years (range, 18–65). Patients were chosen by doctors to be treated in this study because they were either heavily pretreated or had comorbidities disabling them to receive full-intensity conditioning regimen. There were 38 (68%) myeloid malignancies [29 AML (13 in CR1 and 16 CR2), 8 MDS (1 CR1, 2 PR1, 3 stable disease, and 2 in relapse), and 1 CML in CR after blast crisis] and 18 (32%) lymphoid malignancies [9 MM in partial remission (PR), 6 CLL (2 CR1 and 4 PR), and 3 ALL (1 CR1 and 2 CR2)]. Among 45 explored patients for cytogenetics, 24 (53%) showed normal karyotype and 21 (47%) were categorized with "poor prognosis" (11 AML, 4 MM, 3 ALL, 2 MDS, and 1 CML) according to commonly known unfavorable cytogenetics in each underlying disease. Before transplantation, 23 (41%) patients received one treatment line, 22 (39%) two lines, and 11 (20%) more than two lines. For sex matching, 48% of patients were sex-mismatched. For CMV serological status (donor/recipient), 41% were −/−, 25% +/+, 4% +/−, and 30% −/+. For ABO matching, 46% were compatible 29% had major incompatibility and 25% minor incompatibility. The median time between diagnosis and allo-HSCT was 15 months (4–168). All patients received PBSC with a median number of infused CD34+ cells of 6.5 × 106/kg (range, 1.4–17.2). Patient characteristics are shown in Table 1.
Hematopoietic recovery, chimerism results, and response rate
After transplantation, 54 (96%) patients engrafted with a median time to neutrophil recovery and platelet recovery of 16 days (range, 4–86) and 11 days (range, 4–82), respectively. The cumulative incidence of neutrophil recovery at day 60 and 90 after transplantation was 93% [95% confidence interval (CI), 80–97] and 95% (95% CI, 91–98), respectively. The cumulative incidence of platelet recovery at day 60 and 90 after transplantation was 85% (95% CI, 80–90) and 87% (95% CI, 83–92), respectively. Complete donor chimerism, defined as having at least 95% of donor cells, was observed in 90% of evaluated patients at 1 month; it increased to 95% at 4 months and finally reached 100% at 6 months. At day 90 post-allo-HSCT, among 46 (82%) evaluable patients, 31 (67%) were in CR, 7 (15%) were in PR, and 8 (17%) were in less than PR.
Toxicity
The whole procedure was well tolerated by the majority of patients; the most frequent adverse event was infection with 62% of grade ≥II, septicemia of grade ≥II was observed in 27% of patients, and in all other grades, III–IV, adverse events did not reach the 10% level. There were three (5%) Epstein–Barr virus (EBV)-induced lymphoma, one patient died from lymphoma, and two have been treated with rituximab and are in CR at the last follow-up. Details of the different adverse events are shown in Table 2.
Acute and chronic GVHD
Seventeen patients developed acute GVHD ≥grade II (eight grade II, two grade III, and seven grade IV) with a cumulative incidence at 3 months of 31% (95% CI, 25–38). The cumulative incidence of chronic GVHD at 12 months was: 32% (95% CI, 25–39) limited and 6% (95% CI, 2–10) extensive and at 18 months was 34% (95% CI, 27–47) limited and 8% (95% CI, 5–12) extensive.
Overall survival, event-free survival, and relapse incidence
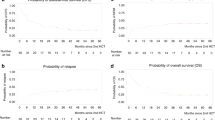
After a median follow-up of 13 months (range, 1–57), the median OS was not reached with a 3-year probability of 52% (95% CI, 38–71), Fig. 1. When we studied the impact of disease status at transplantation on OS, we found a better, but not significant OS rate at 3 years for patients in CR1 (65%) versus those in CR2 (44%; p = 0.25). Patients with active chronic GVHD appeared to benefit from the GVL effect and thus had a better OS rate when studied in univariate analysis [hazard ratio (HR) = 0.2; 95% CI, 0.1–0.6; p = 0.002] with a lower relapse incidence (relapse incidence at 1 year, 15% (95% CI, 14.6–15.4) in patients with active chronic GVHD versus 40.7% (95% CI, 40–41) in patients with no active GVHD, p = 0.06).The median time of EFS was 15 months (range, 8–57) with a 3-year probability of 47% (95% CI, 35–64), Fig. 2. The cumulative incidence of relapse at 3 years after transplantation was 25% (95% CI, 19–31), Fig. 3.
Transplant-related mortality and causes of death
The cumulative incidence of TRM at 12, 24, and 36 months was 20% (95% CI, 16–27) and 23% (95% CI, 16–29) (same at 24 and 36 months), respectively, Fig. 4. At the last follow-up, 22 patients had died, 7 due to relapse and 15 due to TRM (5 pneumonia, 4 GVHD, 1 EBV-induced lymphoma, and 6 other toxicities).
Multivariate analysis
The multivariate analysis studying age, cytogenetics, type of disease, number of previous treatments, sex matching, ABO compatibility, CMV matching, disease status at transplantation, interval diagnosis/allo-HSCT, and CD34+ infused cell number showed only a negative significant impact of minor ABO incompatibility on OS HR = 5.05 (95% CI, 1.8–14); p < 0.001. There was no significant impact of studied factors on both relapse incidence and TRM.
Discussion and conclusion
Our study showed that the combination of treosulfan, fludarabine, and ATG represents a suitable option as a new conditioning regimen leading to promising results in terms of safety and efficacy. Engraftment was achieved in 96% of patients; in terms of toxicity, there was no unexpected adverse event observed within the entire population; infection and septicemia were the most frequent events, and all other adverse events were comparable to those observed in other treosulfan-based allo-HSCT trials [14–22]. We did not observe any increase in hepatic, cardiac, CNS, or pulmonary toxicities as reported in studies that used either MAC or RIC based on busulfan, melphalan, or total body irradiation [11, 29–32]. Nevertheless, we observed three cases of EBV infections that lead to later lymphoma development. The first patient was heavily pretreated for an ALL resistant to different types of chemotherapies, and then received a first allo-HSCT, relapsed 3 months later, and was then included in our study after being put in CR. One month after second allo-HSCT, the patient was EBV+, and the EBV-induced lymphoma diagnosis was confirmed; a few days later, treatment with rituximab was initiated, and then CHOP was added for resistance. The patient died 40 days later from its lymphoma. The second patient was also heavily pretreated for an AML, received induction, then two courses of consolidation chemotherapy, autografted and relapsed 3 years later, put in CR again, was then included in this study. EBV-induced lymphoma appeared 2 months later; a treatment with rituximab has been initiated, and the patient was in CR for his lymphoma at the last follow-up. The last patient was an AML diagnosis who received induction and consolidation chemotherapies, with maintenance of very bad hematopoietic, development of a MDS 2 years later, treated in this study, and developed an EBV-induced lymphoma 3 months later; treatment with rituximab leads to a CR at the last follow-up. This was also observed with a same incidence rate in the prospective study by Casper et al., where two additional fatal cases were observed in the MUD patient's arm [14]. Similarly, EBV-induced lymphoproliferative disorders have been described after allo-HSCT and were observed after T cell depletion [26, 33]; in our case, in vivo ATG was used, and lymphoma development is more attributable to patient history than to our study treatment procedures. Interestingly, despite PBSC as HSC source and MUD, GVHD incidence was acceptable either for acute or for chronic forms, and reached 31% at 3 months and 32% at 1 year, respectively. The use of ATG has probably contributed to a better immunological modulation and leads to a lower GVHD incidence. Mohty et al. assessed the role of ATG in patients receiving allo-HSCT from MUD after myeloablative conditioning for hematological malignancies and found a beneficial effect of its use especially in lowering the GVHD incidence [34]. In survival analyses, we showed a probability of 52% at 3 years; this was not influenced by disease type or its related factors, which suggests that this conditioning regimen can be effective in different types of hematological malignancies with a remarkable GVL effect with the plateau phase beyond 2 years; this was comparable to non-myeloablative conditioning results already described [35]. At 3 years, the relapse incidence reached 25%, and TRM rate was acceptable taking into account the unrelated settings as well as the selected patient population. It was 20% at 1 year and 23% at 3 years; no direct treosulfan-related mortality was observed. Regarding the negative impact of ABO mismatching on OS in our study, we can say that its role in allo-HSCT is still controversial. Klumpp et al. [36] found a lack of effect of ABO mismatching on transplant outcomes while Kanda et al. [37] found in a large meta-analysis a marginally lower OS in recipients of minor ABO mismatched grafts from unrelated donors. In our study, and in view of the small sample size, no significant conclusion about this can be drawn, and large prospective studies are needed to assess this impact. Nemecek et al. [38] evaluated in a prospective study the efficacy and safety of treosulfan 14 g/m2/day i.v. (day −6 to day −4) plus fludarabine 30 mg/m2/day i.v. (day −6 to day −2) in AML, ALL, and MDS patients receiving allo-HSCT from related and unrelated donors. In this study, 60 patients were included, and no difference in outcome was observed between transplantations from MUD or siblings in multivariate analysis. Casper et al. [14] reported results in 55 patients with various hematological malignancies, who were conditioned using treosulfan 10 (n = 20), 12 (n = 18), or 14 (n = 17) g/m2/day i.v. (day −6 to day −4) plus fludarabine 30 mg/m2/day i.v. (day −6 to day −2) and transplanted from either HLA identical or one mismatched sibling donor, or from HLA-identical unrelated donor. They reported higher incidence of grade IV NCI CTC adverse events compared to our results, with 25%, 22%, and 29% for the three studied treosulfan doses, respectively. Grade II–IV aGVHD at day 100 was also higher than what we have observed with 42% including 5% of grade III–IV. At 2 years, the OS probability and relapse incidence for the whole population were 64% and 31%, respectively. Treosulfan, fludarabine, and ATG conditioning combination have been evaluated by Kröger et al. [16] in secondary AML and MDS patients receiving allo-HSCT from related or unrelated donors. Acute grade II–IV GVHD was seen in 23% of patients, and chronic GVHD reached 36%. The 2-year OS and relapse incidence were 36% and 21%, respectively, and the TRM rate was 28% at 100 days. No statistically significant difference was observed between transplants from related and unrelated donors due to the small patient number in the studied arms (n = 6 versus n = 20, respectively). Recently, Blaise et al. evaluated in a retrospective study the outcomes of fludarabine, oral busulfan, and ATG in conditioning allo-HSCT for hematological malignancies from matched HLA siblings [39]. The 5-year OS, PFS, and TRM were 60%, 54%, and 25%, respectively, but a high GVHD incidence was observed with 43% and 81%, respectively, for acute and chronic forms. The authors associated this outcome with an insufficient immunomodulation with the low ATG doses administered. Other busulfan/fludarabine-based conditioning studies in MDS or secondary AML were performed. The German Cooperative Study Group reported 37 patients with MDS and secondary AML transplanted from related or unrelated donors after using fludarabine-, busulfan-, and ATG-based conditioning [40]. The TRM rate reached 45% in the MUD arm and 12% in the sibling arm.
We showed in this prospective phase II study that treosulfan-based conditioning can offer the desired low toxicity of a RIC with a full-intensity antileukemic activity not only to patients ineligible for a standard conditioning regimen either due to increased age or because of other comorbidities but can also be considered a promising alternative treatment for other populations with different hematological malignancies.
References
Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, Chehata S, Esperou H, Vernant JP, Michallet M, Buzyn A, Gratecos N, Cahn JY, Bourhis JH, Chir Z, Raffoux C, Socie G, Golmard JL, Jouet JP (2006) Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol 24(36):5695–5702
Weisdorf DJ, Anasetti C, Antin JH, Kernan NA, Kollman C, Snyder D, Petersdorf E, Nelson G, McGlave P (2002) Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood 99(6):1971–1977
Michallet M, Sobh M, Milligan D, Morisset S, Niederwieser D, Koza V, Ruutu T, Russell NH, Verdonck L, Dhedin N, Vitek A, Boogaerts M, Vindelov L, Finke J, Dubois V, van Biezen A, Brand R, de Witte T, Dreger P (2010) The impact of HLA matching on long-term transplant outcome after allogeneic hematopoietic stem cell transplantation for CLL: a retrospective study from the EBMT registry. Leukemia 24(10):1725–1731
Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, Hurley C, Kollman C, Anasetti C, Noreen H, Begovich A, Hildebrand W, Petersdorf E, Schmeckpeper B, Setterholm M, Trachtenberg E, Williams T, Yunis E, Weisdorf D (2004) Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood 104(7):1923–1930
Dini G, Cancedda R, Locatelli F, Bosi A, Bandini G, Alessandrino EP, Porta F, Uderzo C, Messina C, Fagioli F, Arcese W, Marenco P, Fanin R, Falda M, Soligo D, La Nasa G, Giardini C, Pession A, Scime R, Di Bartolomeo P, Bruno B, Garbarino L, Lamparelli T, Giorgiani G, Lanino E, Manzitti C, Bacigalupo A (2001) Unrelated donor marrow transplantation: an update of the experience of the Italian Bone Marrow Group (GITMO). Haematologica 86(5):451–456
Bierman PJ, Sweetenham JW, Loberiza FR Jr, Taghipour G, Lazarus HM, Rizzo JD, Schmitz N, van Besien K, Vose JM, Horowitz M, Goldstone A (2003) Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin's lymphoma: a comparison with allogeneic and autologous transplantation—The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol 21(20):3744–3753
Anderson JE, Litzow MR, Appelbaum FR, Schoch G, Fisher LD, Buckner CD, Petersen FB, Crawford SW, Press OW, Sanders JE et al (1993) Allogeneic, syngeneic, and autologous marrow transplantation for Hodgkin's disease: the 21-year Seattle experience. J Clin Oncol 11(12):2342–2350
Khouri IF, Keating MJ, Saliba RM, Champlin RE (2002) Long-term follow-up of patients with CLL treated with allogeneic hematopoietic transplantation. Cytotherapy 4(3):217–221
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, Thomas ED (1988) Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 6(10):1562–1568
Balduzzi A, Valsecchi MG, Silvestri D, Locatelli F, Manfredini L, Busca A, Iori AP, Messina C, Prete A, Andolina M, Porta F, Favre C, Ceppi S, Giorgiani G, Lanino E, Rovelli A, Fagioli F, De Fusco C, Rondelli R, Uderzo C (2002) Transplant-related toxicity and mortality: an AIEOP prospective study in 636 pediatric patients transplanted for acute leukemia. Bone Marrow Transplant 29(2):93–100
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R (1998) Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91(3):756–763
Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, Claxton D, Donato M, Bruton J, Cohen A, Davis M, Andersson BS, Anderlini P, Gajewski J, Kornblau S, Andreeff M, Przepiorka D, Ueno NT, Molldrem J, Champlin R (2001) Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 97(3):631–637
Gropp M, Meier W, Hepp H (1998) Treosulfan as an effective second-line therapy in ovarian cancer. Gynecol Oncol 71(1):94–98
Casper J, Wolff D, Knauf W, Blau IW, Ruutu T, Volin L, Wandt H, Schafer-Eckart K, Holowiecki J, Giebel S, Aschan J, Zander AR, Kroger N, Hilgendorf I, Baumgart J, Mylius HA, Pichlmeier U, Freund M (2010) Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol 28(20):3344–3351
Markiewicz M, Wojciechowska M, Wylezol I, Giebel S, Wozniczka K, Wojnar J, Mendek-Czajkowska E, Holowiecki J (2005) First two successful unrelated donor bone marrow transplantations for paroxysmal nocturnal hemoglobinuria in Poland. Ann Transplant 10(3):26–30
Kroger N, Shimoni A, Zabelina T, Schieder H, Panse J, Ayuk F, Wolschke C, Renges H, Dahlke J, Atanackovic D, Nagler A, Zander A (2006) Reduced-toxicity conditioning with treosulfan, fludarabine and ATG as preparative regimen for allogeneic stem cell transplantation (alloSCT) in elderly patients with secondary acute myeloid leukemia (sAML) or myelodysplastic syndrome (MDS). Bone Marrow Transplant 37(4):339–344
Shimoni A, Hardan I, Shem-Tov N, Rand A, Yerushalmi R, Nagler A (2007) Fludarabine and treosulfan: a novel modified myeloablative regimen for allogeneic hematopoietic stem-cell transplantation with effective antileukemia activity in patients with acute myeloid leukemia and myelodysplastic syndromes. Leuk Lymphoma 48(12):2352–2359
Casper J, Knauf W, Kiefer T, Wolff D, Steiner B, Hammer U, Wegener R, Kleine HD, Wilhelm S, Knopp A, Hartung G, Dolken G, Freund M (2004) Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood 103(2):725–731
Schmidt-Hieber M, Blau IW, Trenschel R, Andreesen R, Stuhler G, Einsele H, Kanz L, Keilholz U, Marinets O, Beelen DW, Fauser AA, Volin L, Ruutu T, Uharek L, Fietz T, Knauf W, Hopfenmuller W, Thiel E, Freund M, Casper J (2007) Reduced-toxicity conditioning with fludarabine and treosulfan prior to allogeneic stem cell transplantation in multiple myeloma. Bone Marrow Transplant 39(7):389–396
Blau IW, Schmidt-Hieber M, Leschinger N, Goldner H, Knauf W, Hopfenmuller W, Thiel E, Blau O (2007) Engraftment kinetics and hematopoietic chimerism after reduced-intensity conditioning with fludarabine and treosulfan before allogeneic stem cell transplantation. Ann Hematol 86(8):583–589
Baronciani D, Rambaldi A, Iori AP, Di Bartolomeo P, Pilo F, Pettinau M, Depau C, Mico C, Santarone S, Angelucci E (2008) Treosulfan/fludarabine as an allogeneic hematopoietic stem cell transplant conditioning regimen for high-risk patients. Am J Hematol 83(9):717–720
Bernardo ME, Zecca M, Piras E, Vacca A, Giorgiani G, Cugno C, Caocci G, Comoli P, Mastronuzzi A, Merli P, La Nasa G, Locatelli F (2008) Treosulfan-based conditioning regimen for allogeneic haematopoietic stem cell transplantation in patients with thalassaemia major. Br J Haematol 143(4):548–551
Ruutu T, Volin L, Beelen DW, Trenschel R, Finke J, Schnitzler M, Holowiecki J, Giebel S, Markiewicz M, Uharek L, Blau IW, Kienast J, Stelljes M, Larsson K, Zander AR, Gramatzki M, Repp R, Einsele H, Stuhler G, Baumgart J, Mylius HA, Pichlmeier U, Freund M, Casper J (2011) Reduced-toxicity conditioning with treosulfan and fludarabine in allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes: final results of an international prospective phase II trial. Haematologica 96(9):1344–1350
Lapierre V, Oubouzar N, Auperin A, Tramalloni D, Tayebi H, Robinet E, Kuentz M, Blaise D, Hartmann O, Herve P, Tiberghien P (2001) Influence of the hematopoietic stem cell source on early immunohematologic reconstitution after allogeneic transplantation. Blood 97(9):2580–2586
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18(4):295–304
Zutter MM, Martin PJ, Sale GE, Shulman HM, Fisher L, Thomas ED, Durnam DM (1988) Epstein–Barr virus lymphoproliferation after bone marrow transplantation. Blood 72(2):520–529
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457
Cox DR (1972) Regression models and life tables. J Royal Stat Soc B 34:187–220
de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, Andersson BS, Gajewski J, Couriel D, Cortes J, Donato M, Neumann J, Champlin R, Giralt S (2004) Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood 104(3):865–872
Shimoni A, Hardan I, Shem-Tov N, Rand A, Herscovici C, Yerushalmi R, Nagler A (2007) Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia 21(10):2109–2116
Dasgupta RK, Rule S, Johnson P, Davies J, Burnett A, Poynton C, Wilson K, Smith GM, Jackson G, Richardson C, Wareham E, Stars AC, Tollerfield SM, Morgan GJ (2006) Fludarabine phosphate and melphalan: a reduced intensity conditioning regimen suitable for allogeneic transplantation that maintains the graft versus malignancy effect. Bone Marrow Transplant 37(5):455–461
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE, York RC, Lin LS, Devine SM, Geller RB, Heffner LT, Hillyer CD, Holland HK, Winton EF, Saral R (1996) Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 17(2):225–230
Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM, Jaffe ES, Sale GE, Horowitz MM, Witherspoon RP, Shriner DA, Weisdorf DJ, Kolb HJ, Sullivan KM, Sobocinski KA, Gale RP, Hoover RN, Fraumeni JF Jr, Deeg HJ (1999) Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 94(7):2208–2216
Mohty M, Labopin M, Balere ML, Socie G, Milpied N, Tabrizi R, Ifrah N, Hicheri Y, Dhedin N, Michallet M, Buzyn A, Cahn JY, Bourhis JH, Blaise D, Raffoux C, Esperou H, Yakoub-Agha I (2010) Antithymocyte globulins and chronic graft-vs-host disease after myeloablative allogeneic stem cell transplantation from HLA-matched unrelated donors: a report from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Leukemia 24(11):1867–1874
Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, Woolfrey AE, Chauncey TR, Flowers ME, Mielcarek M, Maloney DG, Storb R (2005) Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 23(9):1993–2003
Klumpp TR, Herman JH, Ulicny J, Emmons RV, Martin ME, Mangan KF (2006) Lack of effect of donor–recipient ABO mismatching on outcome following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 38(9):615–620
Kanda J, Ichinohe T, Matsuo K, Benjamin RJ, Klumpp TR, Rozman P, Blumberg N, Mehta J, Sohn SK, Uchiyama T (2009) Impact of ABO mismatching on the outcomes of allogeneic related and unrelated blood and marrow stem cell transplantations for hematologic malignancies: IPD-based meta-analysis of cohort studies. Transfusion 49(4):624–635
Nemecek ER, Guthrie KA, Sorror ML, Wood BL, Doney KC, Hilger RA, Scott BL, Kovacsovics TJ, Maziarz RT, Woolfrey AE, Bedalov A, Sanders JE, Pagel JM, Sickle EJ, Witherspoon R, Flowers ME, Appelbaum FR, Deeg HJ (2011) Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant 17(3):341–350
Blaise D, Farnault L, Faucher C, Marchetti N, Furst S, El Cheikh J, Ladaique P, Vey N, Bouabdallah R, Stoppa AM, Lemarie C, Calmels B, Prebet T, Castagna L, Chabannon C, Mohty M, Esterni B (2010) Reduced-intensity conditioning with fludarabin, oral busulfan, and thymoglobulin allows long-term disease control and low transplant-related mortality in patients with hematological malignancies. Exp Hematol 38(12):1241–1250
Kroger N, Bornhauser M, Ehninger G, Schwerdtfeger R, Biersack H, Sayer HG, Wandt H, Schafer-Eckardt K, Beyer J, Kiehl M, Zander AR (2003) Allogeneic stem cell transplantation after a fludarabine/busulfan-based reduced-intensity conditioning in patients with myelodysplastic syndrome or secondary acute myeloid leukemia. Ann Hematol 82(6):336–342
Conflict of interest
J Baumgart is an employee of medac GmbH. All the other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michallet, M., Sobh, M., Milpied, N. et al. Phase II prospective study of treosulfan-based reduced-intensity conditioning in allogeneic HSCT for hematological malignancies from 10/10 HLA-identical unrelated donor. Ann Hematol 91, 1289–1297 (2012). https://doi.org/10.1007/s00277-012-1429-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-012-1429-y