Abstract
The clinical efficacy and safety of a four-drug combination of bortezomib, cyclophosphamide, thalidomide, and dexamethasone was assessed for patients with relapsed or refractory multiple myeloma. Seventy patients received at least two cycles of treatment with bortezomib 1.3 mg/m2 intravenously on days 1, 4, 8, and 11; cyclophosphamide 150 mg/m2 orally on days 1–4; thalidomide 50 mg/day orally every day; and dexamethasone 20 mg/m2 intravenously on days 1, 4, 8, and 11. The overall best response rate was 88%, with 46% complete response, 9% very good partial response, and 33% partial response. After a median follow-up of 12.6 months, the median progression-free survival (PFS) was 14.6 months with a 3-year PFS of 14% and the median overall survival (OS) was 31.6 months with a 3-year OS of 47%. Grade 3 or 4 adverse events included thrombocytopenia (12%), neutropenia (4%), peripheral sensory neuropathy (3%), with thrombosis being very rare (<1%). Bortezomib combined with cyclophosphamide, thalidomide, and dexamethasone is a highly effective salvage therapy with manageable toxicity for patients with relapsed or refractory multiple myeloma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma (MM) is a plasma cell malignancy characterized by paraproteinemia, immune paresis, skeletal destruction, renal dysfunction, anemia, and hypercalcemia [1]. Over the past 30 years, there has been a rapid rise in the incidence of MM in Korea, which is likely due to a combination of increased detection, worsening exposure to environmental carcinogens associated with rapid industrialization, and an otherwise aging society, in which overall society has improved. Indeed, MM is emerging as one of the main hematologic malignancies in Korea, and therapeutic approaches to this disease have become especially important. In newly diagnosed disease, conventional combination chemotherapy with alkylating agents and corticosteroids results in less than 5% complete response (CR) [2, 3], Although vincristine, doxorubicin, and dexamethasone (VAD) provides an acceptable response rate, this and similar regimens have several disadvantages, with dexamethasone contributing to most of the activity [4]. Although high-dose chemotherapy with hematopoietic stem cell transplantation (HSCT) induces increased response and prolonged survival [5–8], relapse is ultimately inevitable. Conventional salvage therapies for relapsed or refractory MM have been unsatisfactory in terms of response rate and survival. However, the recent introduction of novel agents, such as bortezomib, thalidomide, and lenalidomide has resulted in clinically significant advances in the treatment of MM [9–12].
Bortezomib (Velcade; Milennium Pharmaceuticals Inc, Cambridge, MA, USA) is a potent first-in-class proteasome inhibitor. Recent clinical trials using bortezomib as a single agent in refractory/relapsed MM, such as SUMMIT, CREST, and APEX, resulted in 30–40% response rates and associated clinical benefits in terms of overall survival [13–15]. In combination with other agents, such as corticosteroids, thalidomide, and chemotherapeutic agents, the response rate increases to 60–80% [9]. With these encouraging results, bortezomib has now been developed as frontline therapy in MM with impressive response rates [16, 17].
Thalidomide has been successfully combined with corticosteroids and alkylating agents in the treatment of relapsed or refractory MM, with response rates ranging from 50–70% [18–22]. Thalidomide in doses from 50–800 mg/day, combined with cyclophosphamide and dexamethasone (CTD), achieves higher response rates (57–79%), with manageable toxicity. However, the depth and quantity of responses from the CTD regimen are unclear, and side effects cumulative, suggesting that further improvement of this regimen is warranted.
This phase 2 trial, therefore, evaluated the efficacy of a four-drug combination of bortezomib, cyclophosphamide, thalidomide, and dexamethasone (Vel-CTD) as salvage treatment in patients with relapsed or refractory MM.
Design and methods
Patients
Eligibility criteria included refractory or relapsed MM patients who must have received at least one prior line of therapy; age 18–75 years; serum M-protein ≥1 g/dL or urine M-protein ≥400 mg/day; Eastern Cooperative Oncology Group performance status ≤2; expected survival ≥6 months; platelet count ≥100 × 109/L; hemoglobin ≥8 g/dL; absolute neutrophil count ≥1.0 × 109/L; aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase less than three times the upper limit of normal; total bilirubin less than two times the upper limit of normal; and creatinine clearance ≥20 mL/min.
Exclusion criteria included prior bortezomib exposure, significant preexisting peripheral neuropathy (defined as greater than or equal to grade 2); uncontrolled or severe cardiovascular disease, pregnancy or breastfeeding, recurrent deep vein thrombosis or pulmonary thromboembolism, significant other co-morbidity such as active ulcer detected at gastroscopy, and radiation therapy within the prior 4 weeks.
The protocol (KMM56) was approved by the institutional review board at each participating center in accordance with the Declaration of Helsinki, and all patients provided written informed consent prior to enrollment.
Study design and treatment schedule
This study was an open-label, non-randomized phase 2 clinical trial conducted at three hospitals in Korea. The primary objectives of this study were to amend response rate and toxicities. Secondary objectives were to determine progression-free survival (PFS) and overall survival (OS).
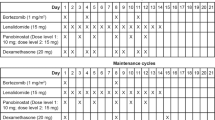
Treatment consisted of 3-week cycles of bortezomib 1.3 mg/m2 intravenously on days 1, 4, 8, and 11; dexamethasone 20 mg/m2/day intravenously on days 1, 4, 8, and 11; cyclophosphamide 150 mg/m2 orally on days 1 to 4; and thalidomide 50 mg orally every day for the entire 21 days. During dexamethasone treatment, trimethoprim/sulfamethoxazole was administrated in order to prevent Pneumocystis carinii infection, while routine antiviral prophylaxis for herpes zoster infection was not administrated. Patients were also given aspirin, 100 mg, to prevent deep-vein thrombosis during thalidomide administration, a proton pump inhibitor prophylactically, and monthly bisphosphonate treatment with zoledronate or pamidronate.
Dose modification and treatment delay
The start of a new cycle could be delayed on a weekly basis (for a maximum of 3 weeks) until recovery of toxicity to a level (grade 2 or less) allowing for the continuation of therapy. Bortezomib was withheld for grade 4 hematological toxicities and grade ≥3 non-hematological toxicities. After adverse events had resolved, bortezomib dose could be reduced from 1.3 to 1 mg/m2 or from 1 to 0.7 mg/m2 according to a previously defined dose reduction algorithm [23]. If a patient had peripheral neuropathy of grade 1 with pain or grade 2, the bortezomib was reduced to 1 mg/m2. For grade ≥3 peripheral neuropathy, the bortezomib was withheld until the peripheral neuropathy resolved to baseline and then restarted at 0.7 mg/m2. Simultaneously, thalidomide was omitted until the toxicity resolved to baseline or decreased to below grade 1. Thalidomide was discontinued permanently in the event of thrombosis despite the prophylaxis
Response assessment
The tumor response was evaluated every two cycles during Vel-CTD therapy. After four cycles, patients with progressive disease were dropped. Subsequently, four additional cycles of Vel-CTD were given unless tumor progression or unacceptable side effects occurred. If the patients did not achieve CR after eight cycles of chemotherapy, they received additional cycles of treatment. Patients were removed from study if disease progressed. To evaluate response, International Myeloma Working Group uniform response criteria were used [24], but, we did not discriminate between CR and stringent CR because the absence of clone cells in bone marrow could not be confirmed. Adverse events were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (v3.0, 2003).
Evaluation of survival
PFS was defined from the time of first treatment to the time of the first sign of disease progression or death. OS was measured from the time of first treatment to the time of the last follow-up or death.
Statistical analysis
Categorical data and continuous variables were assessed using Fisher' exact test and the Mann–Whitney U test, respectively. OS and PFS were analyzed using Kaplan–Meier survival curve estimates. P < .05 was considered statistically significant, and 95% confidence intervals were eliminated accordingly. All statistical computations were performed using Statistical Package for the Social Sciences ver. 17.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
Seventy patients with relapsed or refractory MM were enrolled between November 2004 and November 2008. The baseline characteristics of the patients are shown in Table 1. There were 35 men (50%) and 35 women (50%). The median age of the patients was 64 years (range 39–76 years). According to the International Staging System, 60%, 21%, and 10% of the patients were stage I, II, and III at the time of initial therapy, respectively. Fluorescence in situ hybridization (FISH) study for deletion 13q14 was available in 56 patients. The median time from diagnosis was 1.5 years (range 0.1–18.9 years). The median number of previous lines of treatment was 2 (range 1–6). Forty (57%) and 34 (49%) patients had prior treatment with CTD and TD, respectively. Thirteen (19%) of the patients had undergone a previous autologous HSCT with high-dose chemotherapy. The median follow-up time was 12.6 months (range 1.7–49.6 months).
Disease response
The median number of treatment cycles with Vel-CTD was 8 (range 2–22). The total number of cycles delivered was 601. Sixty-four (91%) and forty (57%) patients received at least four or eight cycles of Vel-CTD, respectively. Five patients discontinued treatment early due to progression, in three patients after three cycles of chemotherapy, and follow-up loss in two patients.
Of the 70 patients, 52 (74%) achieved partial response (≥PR) after the first two cycles of Vel-CTD. For the 64 patients who completed four cycles of therapy, the overall response rate (ORR: PR or better) was 81%, including 31% CR, 14% very good partial response (VGPR), and 36% PR. For the 40 patients who completed eight cycles of therapy, the ORR was 91%, including 43% CR, 13% VGPR, and 35% PR. When we analyzed the best response for the 70 patients who received ≥2 cycles of Vel-CTD, 61 patients (88%) achieved a response, including 32 (46%) CR, six (9%) VGPR, and 23 (33%) PR (Fig. 1). There was no significant difference in the CR and ORR rate according to the presence or absence of deletion 13q14 by FISH analysis.
Survival and prognostic factors
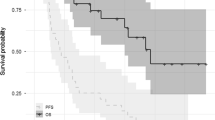
With a median follow-up of 12.6 months, the median OS was 31.6 months (95% confidence interval (CI): not evaluable), and the estimated OS at 3 years was 47 ± 9%. The median PFS was 14.6 months (95% CI: 13.4–15.8), and the PFS at 3 years was 14 ± 7% (Fig. 2). Patients who achieved a good response (≥PR) after four cycles of Vel-CTD had a significantly longer OS and PFS than those with poor therapeutic responses (≤SD; OS not reached vs. 12.9 months, P = .010; PFS 17.3 vs. 7.5 months, P < .001; Fig. 3a and b). Furthermore, patients who achieved a good response (≥PR) after eight cycles of Vel-CTD also had a significantly longer PFS than those with poor therapeutic responses (≤SD; OS not reached vs. 12.7 months, P = .006; PFS 17.3 vs. 4.5 months, P < .000; Fig 3c and d). When we analyzed other factors affecting survival, the presence of deletion 13q14 was associated with a shorter PFS in patients treated with Vel-CTD than in those without this abnormality (10.1 months vs. 16.3 months, P = .031). While the OS in these patients seemed to show a similar trend, there was no statistical significance (31.6 months vs. 16.0 months, P = .110; Fig. 4).
Response to Vel-CTD in patients with prior HSCT with high-dose chemotherapy
Thirteen (19%) patients had undergone high-dose chemotherapy and autologous HSCT prior to Vel-CTD treatment. All of these patients received at least four cycles of Vel-CTD. Among these patients, the ORR was 77%, including 46% CR, 8% VGPR, and 23% PR. The median OS and PFS were 27.3 months (95% CI: 6.6–48.0) and 14.7 months (95% CI: 8.4–21.0), respectively.
Adverse events
Of the 601 evaluable cycles delivered, grade 3–4 hematological toxicities including thrombocytopenia (12%), neutropenia (4%), and anemia (4%) were observed (Table 2). Non-hematological toxicities are also listed in Table 2. The most frequently observed grade 3/4 non-hematologic toxicity was fatigue (4%), including 1% grade 4 toxicity. Treatment-induced peripheral sensory neuropathy was grade 1 to 3 in 17%, 15%, and 3% of the delivered cycles, respectively. Most of the patients were also given gabapentin (300–1,800 mg a day), non-steroidal anti-inflammatory drugs or opioids as adjuvant drugs for pain control, depending on pain severity. Gastrointestinal toxicity was mainly due to constipation related to thalidomide, with most patients responding to laxatives.
Of the 70 patients, ten (14%) developed pneumonic infiltrations, including four patients with multiple episodes (Table 3). Of these, two patients (3%) died of pneumonia and sepsis. One episode of sepsis developed during a neutropenic period, whereas the other episode was not associated with neutropenia. Nine patients (13%) developed herpes zoster, especially during the early treatment course (cycles 1–3), suggesting that viral prophylaxis during the early treatment cycles is warranted as previously reported [25]. Symptomatic pulmonary thromboembolism also occurred in one patient (1%) after two cycles of chemotherapy. She continued on Vel-CD treatment without thalidomide, an appropriate anticoagulate, and had no further episodes.
Discussion
Our phase 2 clinical trial evaluated the clinical efficacy and safety of the Vel-CTD regimen in patients with relapsed or refractory MM. This four-drug combination of bortezomib, cyclophosphamide, thalidomide, and dexamethasone proved a highly effective salvage regimen in terms of response rate, with an 88% overall best response rate, including 46% in CR. Responses were also rapid and durable with a 74% ORR after the first two cycles, and a PFS of 14.6 months in PFS. The efficacy of Vel-CTD in our study was superior to the results of a similar four-drug combination of bortezomib, melphalan, dexamethasone, and thalidomide (VMPT) where, the ORR and CR rates of the VMPT regimen were 66–67% and 13–17%, respectively [26, 27]. Achieving CR is considered to be an important surrogate for improving the survival of MM patients. In our study, Vel-CTD induced a higher CR rate than other four-drug combinations. Furthermore, as shown in previous studies [8, 28], the regimen was an effective salvage therapy in MM patients who had relapsed after auto-HSCT.
Although there were several differences among studies, such as different doses of bortezomib, thalidomide, corticosteroid, and different alkylating agents with melphalan or cyclophosphamide, the main difference may be the dose-intensity of bortezomib. In our study, bortezomib was administered four times at 1.3 mg/m2 dosage in each 3-week cycle (1.73 mg/m2/week). By contrast, in two studies of VMPT, bortezomib was administered four times at 1.0 mg/m2 in a 4-week cycle (1.0 mg/m2/week) in one study and four times at 1.3 mg/m2 in a 5-week cycle (1.04 mg/m2/week) in the other [26, 27].
Bortezomib was recently approved for the treatment of relapsed or refractory MM based on large studies, including SUMMIT, CREST, and APEX. These trials demonstrated 30–40% response rates, including 10% CR or near CR (nCR) as a single agent in a salvage setting [13–15]. As a combination with other targeted therapies, such as thalidomide and/or chemotherapeutic agents, response rates increase to 50%, including 20% CR. Indeed, a previous study using a triple-drug combination of bortezomib, melphalan, and dexamethasone showed considerable response rates, including a 76% ORR with a 34% CR/nCR at the maximum tolerated dose [29]. Reece et al. [30] assessed the efficacy of the combination of bortezomib, cyclophosphamide, and prednisone and observed a CR in more than 50%.
In terms of treatment-related toxicities, side effects such as sensory neuropathy and constipation were generally manageable with dose reduction of bortezomib and/or thalidomide as well as appropriate supportive care. Although the greater incidence of neuropathy using the combination of bortezomib and thalidomide was a concern, Vel-CTD using low-dose thalidomide at 50 mg daily had a low incidence (3%) of grade 3–4 neuropathy, which was similar to the incidence in a previous study using the same dose of thalidomide in VMPT [27]. However, some patients suffered from infectious complications, such as pneumonia or herpes zoster, especially early in their treatment (cycles 1–3), indicating the need for careful observation and viral prophylaxis early in the treatment course.
In conclusion, the Vel-CTD regimen appears generally well-tolerated and has significant activity in patients with relapsed or refractory MM, with this combination warranting further evaluation in future trials.
References
Kyle RA, Rajkumar SV (2004) Multiple myeloma. N Engl J Med 351:1860–1873
Galton DA, Peto R (1968) A progress report on the Medical Research Council's therapeutic trial in myelomatosis. Br J Haematol 15:319–320
Sirohi B, Powles R (2004) Multiple myeloma. Lancet 363:875–887
Alexanian R, Barlogie B, Tucker S (1990) VAD-based regimens as primary treatment for multiple myeloma. Am J Hematol 33:86–89
Barlogie B, Jagannath S, Desikan KR et al (1999) Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood 93:55–65
Samson D, Gaminara E, Newland A et al (1989) Infusion of vincristine and doxorubicin with oral dexamethasone as first-line therapy for multiple myeloma. Lancet 2:882–885
Sirohi B, Powles R, Mehta J et al (1999) Complete remission rate and outcome after intensive treatment of 177 patients under 75 years of age with IgG myeloma defining a circumscribed disease entity with a new staging system. Br J Haematol 107:656–666
Goldschmidt H, Hegenbart U, Wallmeier M et al (1997) Factors influencing collection of peripheral blood progenitor cells following high-dose cyclophosphamide and granulocyte colony stimulating factor in patients with multiple myeloma. Br J Haematol 98:736–744
Richardson PG, Hideshima T, Mitsiades C et al (2007) The emerging role of novel therapies for the treatment of relapsed myeloma. J Natl Compr Canc Netw 5:149–162
San Miguel JF, Schlag R, Khuageva NK et al (2008) Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 359:906–917
Dimopoulos M, Spencer A, Attal M et al (2007) Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 357:2123–2132
Weber DM, Chen C, Niesvizky R et al (2007) Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 357:2133–2142
Richardson PG, Barlogie B, Berenson J et al (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348:2609–2617
Jagannath S, Barlogie B, Berenson J (2004) A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 127:165–172
Richardson PG, Sonneveld P, Schuster M et al (2007) Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood 110:3557–3560
Wang LM, Weber DM, Delasalle KB et al (2004) VTD (Velcade, thalidomide, dexamethasone) as primary therapy for newly diagnosed multiple myeloma. Blood 104:210a
Oakervee HE, Popat R, Curry N et al (2005) PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol 129:755–762
Dimopoulos MA, Zervas K, Kouvatseas G et al (2001) Thalidomide and dexamethasone combination for refractory multiple myeloma. Ann Oncol 12:991–995
Weber DM, Rankin K, Gavino M et al (2000) Thalidomide with dexamethasone for resistant multiple myeloma. Blood 96:167a
Palumbo A, Giaccone L, Bertola A et al (2001) Low-dose thalidomide plus dexamethasone is an effective salvage therapy for advanced myeloma. Haematologica 86:399–403
Garcia-Sanz R, Gonzalez-Fraile MI, Sierra M, Lopez C et al (2003) The combination of thalidomide, cyclophosphamide and dexamethasone (ThaCyDex) is feasible and can be an option for relapsed/refractory multiple myeloma. Hematol J 3:43–48
Srkalovic G, Elson P, Trebisky B et al (2002) Use of melphalan, thalidomide, and dexamethasone in treatment of refractory and relapsed multiple myeloma. Med Oncol 19:219–226
Richardson PG, Briemberg H, Jagannath S et al (2006) Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 24:3113–3120
Durie BG, Harousseau JL, Miguel JS et al (2006) International uniform response criteria for multiple myeloma. Leukemia 20:1467–1473
Chanan-Khan A, Sonneveld P, Schuster MW et al (2008) Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol 26:4784–4790
Terpos E, Kastritis E, Roussou M et al (2008) The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia 22:2247–2256
Palumbo A, Ambrosini MT, Benevolo G et al (2007) Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood 109:2767–2772
Musto P, Falcone A, Sanpaolo G et al (2006) Bortezomib (Velcade) for progressive myeloma after autologous stem cell transplantation and thalidomide. Leuk Res 30:283–285
Popat R, Oakervee H, Williams C et al (2009) Bortezomib, low-dose intravenous melphalan, and dexamethasone for patients with relapsed multiple myeloma. Br J Haematol 144:887–894
Reece DE, Rodriguez GP, Chen C et al (2008) Phase I-II trial of bortezomib plus oral cyclophosphamide and prednisone in relapsed and refractory multiple myeloma. J Clin Oncol 26:4777–4783
Acknowledgments
The authors thank Dr. Paul Richardson, Dana-Faber Cancer Institute, for critically review the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YK., Sohn, SK., Lee, JH. et al. Clinical efficacy of a bortezomib, cyclophosphamide, thalidomide, and dexamethasone (Vel-CTD) regimen in patients with relapsed or refractory multiple myeloma: a phase II study. Ann Hematol 89, 475–482 (2010). https://doi.org/10.1007/s00277-009-0856-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-009-0856-x