Abstract
The evidence that leukocytes may contribute to the pathogenesis of thrombosis in Chronic Myeloproliferative Neoplasms is increasing but not definitive. To further enforces whether an increased leukocyte count is associated with thrombosis and whether this effect can be modulated by cytoreductive therapy, we analyzed the clinical course of 187 patients with Polycythemia Vera (PV) and Essential Thrombocythemia (ET) followed at two Italian Institutions over a period of 7 years. The association was measured at diagnosis or before thrombotic events: a multivariable analysis was carried out using data at baseline and time-dependent covariates. We found that white blood cells (WBC) count above 9.5 × 109/L at diagnosis (baseline analysis) was associated with thrombosis during the follow-up (Hazard Ratio [HR] of 1.8, p 0.03). At the time-dependent analysis, therapy with hydroxyurea (HU), lowering by 35% the baseline WBC level, reduced such strength of association giving a HR of 1.3 (p value non significant). We found a trend between WBC level and thrombosis in untreated low-risk patients (RR of 1.9, 95% CI 0.9 to 3.1); in high-risk patients treated with HU this correlation was clearly lost (RR 1.1, 95% CI 0.2 to 2.7). Finally, we could not identify the presence of JAK2 V617F as a risk factor for thrombosis. Properly designed prospective studies should corroborate such results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Ph-negative myeloproliferative neoplasms (MPNs; namely polycythemia vera [PV], essential thrombocythemia [ET] and primary myelofibrosis [PMF]) are characterized by clonal proliferation of hematopoietic precursors leading to an increased production of erythrocytes as well as leukocytes and platelets. These diseases are usually complicated by thrombosis, with a rate of major thrombosis as high as about 50% [1–4]. Advanced age and a prior history of thrombosis are the two most important risk factors for vascular complications, while hyper-cholesterolemia, hypertension, smoking, and diabetes have been recognized as predictors of thrombosis [1–4]. In the last few years, new information on the pathogenesis of thrombosis in MPNs became available, including the role of leukocyte activation and interaction with platelets as well as the presence of JAK2 V617F mutation [5, 6]. Very recently, leukocytosis has been reported as an independent risk factor for thrombosis in both PV and ET [7–10] and this is particularly true in “low-risk” patients who normally do not require cytoreductive therapy. However, additional data are required to firmly establish the role of such predictors for thrombosis as well as their clinical advantage. We analyzed the prognostic role of white blood cell values for developing thrombosis in a cohort of patients affected by PV and ET.
Materials and methods
Patients
Our population consisted of 187 patients with ET (88) and PV (99) regularly followed-up at two Hematology Divisions from 2000 to 2007. Polycythemia vera and ET were diagnosed according to Polycythemia Vera Study Group (PVSG) criteria [11] or by Pearson et al. [12]; only patients with confirmed diagnosis were included in the follow-up. The time elapsed from diagnosis to referral is reported in Table 1. Data regarding laboratory values, treatments, and clinical outcomes were collected at diagnosis and every 6 months during the follow-up. JAK2 V617F mutation was assessed in 78 cases (41.3%). Patients were classified as being at low or high risk for thrombosis according to standard risk factors (age 60 years and/or a previous major thrombotic event) and managed accordingly [13]. Treatment strategies (based on hydroxyurea [HU], anagrelide or α-Interferon) had to comply with recommendation of maintaining hematocrit below 0.45 and the platelet below 400 × 109/L for patients with PV and platelets below 600 × 109/L in patients with TE
Diagnosis of thrombosis
The thrombotic events had to be occurred after referral to our hematology center or in the 6 months preceding the diagnosis. Vascular events included ischemic stroke, cerebral transient ischemic attack (TIA), acute myocardial infarction (AMI), peripheral arterial thrombosis (PAT), and venous thromboembolism (VTE). Diagnostic procedures for establishing thrombosis included cerebral computed tomography (CT) or magnetic resonance imaging for stroke, characteristic neurologic symptoms for TIA, electrocardiography and/or increased cardiac enzymes for AMI, angiography for PAT, and ultrasonography of the arms or legs or pulmonary ventilation−perfusion scan or CT scan for VTE. The median duration of the follow-up was 3.2 years (0.4−7 years).
JAK2 V617F mutation analysis
DNA was extracted from peripheral blood of 187 patients at diagnosis. Allele-specific PCR for the detection of JAK2 V617 F was performed on a ABI 7900 HT real time PCR system (Applied Biosystems) using specific Taqman primers for the mutated allele and for the non-mutated sequence as previously described [14].
Statistical methods and ethics
We performed univariate and multivariate analyses. The first was performed for evaluating differences in proportions by the chi-square and Fisher exact tests, while differences in continuous variables were tested by the t test. Two different multivariable approaches were used. Firstly, a multivariable analysis using values measured at diagnosis was used to assess whether the level of exposition for a potential risk predictor, evaluated at diagnosis, could be found to be a statistically significant marker of increased probability of recurrence of thrombosis during the follow-up. The relative multivariable model has been fitted after adjusting for sex, standard risk factors for thrombosis (age ≥ 60 years and/or previous thrombosis) and median levels of white blood cell count, hemoglobin level, hematocrit, and platelet count registered at diagnosis. Secondly, the database was explored using Cox proportional-hazards model with time-varying covariates to evaluate risk and with right censoring at the first thrombotic event or last date of follow-up. This analysis included fixed (sex and standard risk factors) and time-varying variables (treatments and median levels of hemoglobin, hematocrit, white blood cells [WBC], and platelets).
The study was conducted according to good clinical and laboratory practice rules and the principles of the Declaration of Helsinki. Informed consent was obtained for each subject.
Results
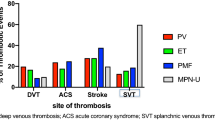
The characteristics of the 187 patients are shown in Table 1. Fifty-nine episodes of vascular thrombosis were registered at the time of diagnosis or in the six previous months (31.5%). During the follow-up, 14 additional cases occurred; totally 73 thrombotic events had occurred (39.0 %). The distribution of events is reported in Table 1.
Parameters at diagnosis associated with thrombosis registered in the follow-up (at univariate analysis) were age ≥ 60 years and previous history of thrombosis (p 0.02) and leukocyte count above the median level of 9.5 × 109/L (p 0.03). No other significant associations were found regarding arterial and venous thrombosis evaluated separately, sex, median levels of hemoglobin, hematocrit, and platelet count.
Accordingly to previous studies [8, 9], two statistical multivariable analyses were performed: (a) the baseline analysis considered the variable at diagnosis (taking in account sex, standard risk factors for thrombosis and median levels of hemoglobin, hematocrit, platelets, WBC). This analysis showed a significant association between standard risk factors and high levels of WBC with the occurrence of thrombosis in the follow-up. (b) The “time-dependent” analysis considered covariates taking into account the last value of blood cell counts measured before vascular events (Table 2). In such population, conventional risk factors for thrombosis maintained their value; however, considering HU influence, the role of WBC for determining thrombosis was lost in patients in whom cytoreductive therapy significantly reduced leukocyte median value by at least 35% from the baseline level of 9.5 × 109/L. In both the analyses, high platelet count did not show any correlation with an increased risk for thrombosis. The association between leukocytosis and thrombosis was considered separately in PV and ET patients, producing a Hazard Ratio (HR) of 1.6 (95% CI 0.4 to 2.8, p 0.07) and of 1.7 (95% CI 0.5 to 3.1, p 0.09), respectively.
We also evaluated the interaction between conventional risk categories and WBC levels accordingly to risk profile. In low-risk patients, a trend (but not significant) was found between high WBC and thrombosis (relative risk [RR] 1.9, 95% CI 0.9 to 3.1); in high-risk patients, this correlation was clearly lost (RR 1.1, 95% CI 0.2 to 2.7).
Considering the role of JAK2 V617F mutation, this was evaluated in 78 patients (46 PV and 32 ET); either the multivariate or univariate analysis did not found this mutation to be a significant predictor for thrombosis (HR 0.8, 95% CI 0.4 to 1.9).
Discussion
Our study shows that in patients with MPNs, an increased number of WBCs is a relevant marker of thrombosis; this characteristic is lost when effective cytoreductive therapy decrease leukocytes of about 35% from the baseline median level. These results confirm previous reports in patients with PV and ET.
Carobbio et al. in a population of 439 ET patients found that an increased leukocyte count at diagnosis (median level of 8.7 × 109/L) was associated with thrombosis during follow-up (“baseline analysis”, RR 2.3, 95% CI 1.4 to 3.9, p 0.001) [8]. Therapy with HU lowered leukocytosis and reduced the strength of the association between leukocytosis and thrombosis (“time-dependent analysis”, RR 1.6, 95% CI 0.9−2.0, not significant). The association of leukocytosis and thrombosis was more evident in untreated low-risk patients (RR 2.7, 95% CI 1.2-6.4, p 0 .01) compared with treated high-risk patients (RR 1.6, 95% CI 0.8−3.2). Finally, the presence of JAK2 V617F was not identified as a risk factor for thrombosis during follow-up despite a significant association between the mutation and leukocytosis.
Landolfi et al. evaluated 1,638 polycythemic patients [9]. The time-dependent analysis adjusted for potential confounders showed that patients with a white blood cell count above 15 × 109/L, compared with those with a white blood cell count below 10 × 109/L, had a significant increase in the risk of thrombosis (HR, 1.71; 95% CI, 1.10−2.65; p 0.017), mainly deriving from an increased risk of myocardial infarction (HR, 2.84; 95% CI, 1.25−6.46; p 0.013). Authors’ conclusions were that leukocyte count may help in defining the vascular risk of polycythemic subjects.
Very recently, Kundranda et al., in a retrospective analysis of 239 patients (180 with PV and 59 with ET) found that WBC and platelet counts were found to be positively associated with the presence of thrombosis (p 0.001) [10]. The proportion of patients with thrombosis for the group with leukocyte count above 10 × 109/L were significantly higher than that for the group with WBC counts < 10 × 109/L. There was also a significant association between the presence of JAK2 V617F mutation and thrombosis.
Wolanskyj et al., in patients with ET, reported that age, previous thrombosis, and leukocytosis were independent risk factors for both major thrombosis and a poorer long-term survival when evaluated at diagnosis [4]. Such results were further confirmed by Gangat et al. in a population of 459 PV patients [15].
In all these investigations except one [10], platelet number did not show any correlation with the vascular events and the contribution of platelet number to major thrombotic events remains poorly established.
The thrombogenic role of WBCs in MNPs has been explained by several investigations that showed that in these disorders, neutrophils circulate in an activated state and are able to bind to platelets in a dynamic adhesive process, which reflects the activation of both platelets and leukocytes [5, 6].
The role of JAK2 V617F, mutation as independent factor for thrombosis is still debated [7, 16–20]. In our series, we did not find any significant role of JAK2 V617F for predicting thrombosis, but some limit of our analysis should be considered because we cannot exclude that this may reflect an inadequate sample size.
According to our and others results, patients previously classified as “low-risk” but carrying high WBC level might be considered for cytoreductive therapy because of the intrinsic high risk for thrombosis [21]. However, the best leukocyte cut-off values for predicting these events still require clinical validation.
In clinical practice, we need to be cautious. First of all, these results did not come from prospective studies and some potential bias may be present. Then, some investigations did not find correlation between high WBC level and thrombosis. Recently, Gangat et al. evaluated 407 “low-risk” patients (153 with PV and 254 with ET) [22]; leukocyte count, considered as either a continuous or categorical variable (using cut-off levels of 15 × 109/L for PV and either 15 × 109/L or 9.4 × 109/L for ET), was not significantly associated with either arterial or venous thrombosis. However, direct comparison across these studies is not easy to attempt.
In conclusion, the association between leukocytosis and thrombosis appears to be a clinically important issue in patients with MPNs, especially in those who usually do not require cytoreductive therapy. The clinical value of such results needs to be confirmed in prospective properly designed trials.
References
Elliott MA, Tefferi A (2005) Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br J Haematol 128:275–90
Marchioli R, Finazzi G, Landolfi R et al (2005) Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 23:2224–32
Passamonti F, Rumi E, Pungolino E et al (2004) Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 117:755–61
Wolanskyj AP, Schwager SM, McClure RF et al (2006) Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 81:159–66
Falanga A, Marchetti M, Barbui T et al (2005) Pathogenesis of thrombosis in essential thrombocythemia and polycythemia vera: the role of neutrophils. Semin Hematol 42:239–247
Arellano-Rodrigo E, Alvarez-Larran A, Reverter JC et al (2006) Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica 91:169–175
Wolanskyj AP, Lasho TL, Schwager SM et al (2005) JAK2V617F mutation in essential thrombocythaemia: clinical implications and long-term prognostic relevance. Br J Haematol 131:208–213
Carobbio A, Finazzi G, Guerini V et al (2007) Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors and Jak2 mutation status. Blood 109:2310–3
Landolfi R, Di Gennaro L, Barbui T et al (2007) Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 109:2446–52
Kundranda MN, Maiti B, Iqbal N et al (2008) The association of leukocytosis, thrombocytosis and JAK2V617F mutation with thrombotic events in myeloproliferative disorders (MPD’s). Blood 112:2803 (Abs)
Murphy S, Peterson P, Iland H et al (1997) Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival and leukemic transition by treatment. Semin Hematol 34:29–39
Pearson TC, Messinezy M, Westwood N, et al. (2000). A polycythemia vera updated: diagnosis, pathobiology, and treatment. Hematology Am Soc Hematol Educ Program 51-68
Barbui T, Finazzi G (2005) When and how to treat essential thrombocythemia. New Engl J Med 353:85–86
Baxter EJ, Scott LM, Campbell PJ et al (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054–1061
Gangat N, Strand J, Li CY et al (2007) Leucocytosis in polycythaemia vera predicts both inferior survival and leukaemic transformation. Br J Haematol 138(3):354–8
Campbell PJ, Scott LM, Buck G et al (2005) Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 366:1945–1953
Cheung B, Radia D, Pantelidis P et al (2005) The presence of the JAK2 V617F mutation is associated with a higher haemoglobin and increased risk of thrombosis in essential thrombocythaemia. Br J Haematol 132:244–250
Antonioli E, Guglielmelli P, Pancrazzi A et al (2005) Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 19:1847–1849
Dahabreh IJ, Zoi K, Giannouli S et al (2009) Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res 33(1):67–73
Ziakas PD (2008) Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica 93:1412–1414
Carobbio A, Antonioli E, Guglielmelli P et al (2008) Leukocytosis and risk stratification assessment in essential thrombocythemia. J Clin Oncol 26(16):2732–6
Gangat N, Wolanskyj A, Schwager S et al (2008) Leukocytosis at diagnosis and the risk of subsequent thrombosis in low-risk essential thrombocythemia and polycythemia vera. Blood 112:1751 (Abs)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caramazza, D., Caracciolo, C., Barone, R. et al. Correlation between leukocytosis and thrombosis in Philadelphia-negative chronic myeloproliferative neoplasms. Ann Hematol 88, 967–971 (2009). https://doi.org/10.1007/s00277-009-0706-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-009-0706-x