Abstract
The aim of this epidemiologic population survey was to assess the penetrance of the most frequent hemochromatosis (HFE) gene variants in ethnic Danish men. A cohort of 6,020 men aged 30–53 years was screened for HFE C282Y, H63D, and S65C variants by restriction fragment length polymorphism analysis. Subsequently, iron status markers (serum transferrin saturation, serum ferritin) were analyzed in 1,452 men. The C282Y allele was present in 5.6%, H63D in 12.8%, and S65C in 1.8% of the men. We found 23 out of 6,020 (0.38%) C282Y homozygotes, of whom two had been treated with phlebotomy. Among untreated C282Y homozygotes (n = 21) with available iron status markers (transferrin saturation n = 18, ferritin n = 16), 89% had elevated transferrin saturation ≥50%, 94% had elevated ferritin ≥300 μg/L, and 88% had elevation of both iron status markers; seven out of 16 (44%) had ferritin values >800 μg/L. One C282Y homozygote had normal iron status markers possibly due to nonexpressivity. Among C282Y/H63D compound heterozygotes (n = 66), 23% had elevated transferrin saturation, 27% elevated ferritin, and 9% elevation of both iron status markers. Among H63D/H63D homozygotes (n = 74), 15% had elevated transferrin saturation, 19% elevated ferritin, and 5.4% elevation of both iron status markers. Among C282Y/wild type (wt) heterozygotes (n = 255), 9% had elevated transferrin saturation, 9% elevated ferritin, and 1.2% elevation of both iron status markers. Among H63D/wt heterozygotes (n = 600), 8% had elevated transferrin saturation, 12% elevated ferritin, and 2% elevation of both iron status markers. None of the men with the S65C variant displayed elevation of both iron status markers. In conclusion, this study demonstrates a high penetrance of the C282Y variant in Danish men followed by the H63D variant while the S65D variant had no significant impact on iron status markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hereditary HFE hemochromatosis is a frequent genetic disorder in individuals of northern European heritage with a significant morbidity and mortality unless adequately treated [1]. Since the discovery of the HFE gene, HFE allelic frequencies have been analyzed in most European populations [2] and screening for hemochromatosis based on phenotypic or genetic testing has been discussed. HFE hemochromatosis is inherited as an autosomal recessive trait [3] and the penetrance of the most significant HFE mutation, i.e., C282Y, has been assessed using phenotypic [4–8] or clinical criteria [9, 10]. Three longitudinal studies have estimated the proportion of C282 homozygous subjects who develop disease due to iron overload [5, 11, 12]. Two studies were underpowered to assess disease prevalence [5, 11]. The largest prospective study comprised ∼200 homozygous individuals followed up for more than 10 years [12].
The aim of this study was to assess the penetrance of the HFE mutations or variants C282Y (c845 G→A), H63D (c187 C→G), and S65C (c193 A→T) on the phenotypic expression of iron status markers in Danish men.
Materials and methods
Participants and study design
This prospective, epidemiologic population survey was performed in 2000–2001 [13, 14]. It was approved by the Scientific Ethical Committee and fulfilled the Declaration of Helsinki. All men of ethnic Danish origin aged 30–53 years (n = 10,993) being residents in Næstved and Vordingborg municipalities in the southern part of Zealand were drawn from the Census Registry and invited by letter to participate. Written informed consent was obtained from all participants.
In the first screening, HFE genotypes were assessed on mailed saliva samples from the participants. The second screening comprised: (1) participants in whom the genotype could not be determined on saliva samples, they were invited to genetic testing and assessment of iron status markers; (2) participants with established HFE variants, they were invited to assessment of iron status markers. Subsequently, participants with elevated iron status markers were invited to a repeat analysis.
HFE variant analysis
Extraction of DNA from saliva and blood was performed by restriction fragment length polymorphism [13–15]. Three cases of C282Y and H63D in “cis” phase have been reported [16–18]. These genotypes were not found in our series and we assume that all examined chromosomes carry only one of the three HFE variants. The three most frequent HFE genotypes C282, H63, and S65 are designated wild type (wt).
Iron status
Blood samples were drawn in the fasting state (except in 250 men) between 0800 and 1000 hours. Serum was stored at 4°C and analyzed within 4 days. Analyses of iron status markers were performed according to the manufacturers’ instructions on Dimension® RxL Clinical Chemistry System with heterogeneous Immunoassay Module (Dade Behring, Deerfield, IL, USA, now merged with Siemens Healthcare Diagnostics, Eschborn, Germany). Serum iron was analyzed with Dimension® IRN Flex reagent catalogue number DF49A using calibrator catalogue number DC21. The normal range for serum iron in men is 13–36 μmol/L. Serum transferrin (molecular weight 78,000 Da) was analyzed with Dimension® TRNF Flex reagent catalogue number DF103 using calibrator catalogue number DC51. The calibrator is standardized according to the International Federation of Clinical Chemistry, International Reference Preparation for Plasma Proteins, the Community Bureau of Reference, and the College of American Pathologists. The normal range for serum transferrin is 24–41 μmol/L.
The serum transferrin saturation percent (TSAT) was calculated by the equation:
The normal range for transferrin saturation is 16–49%; we consider values ≥50% to be elevated [19].
Serum ferritin was analyzed with Dimension® FERR Flex reagent catalogue number RF440 using calibrator catalogue number RC440. The calibrator is standardized according to the World Health Organization (WHO) ferritin standard, 3rd IS, 95/572 [20]. We used the following cut-off values: normal range for ferritin, 15–299 μg/L; elevated ferritin, ≥300 μg/L; moderate iron overload for ferritin, 300–800 μg/L; major iron overload for ferritin, >800 μg/L [19, 21].
Statistics
Statistical analyses were performed using the Statistical Package for the Social Sciences 11.5.1 for Windows. Due to the non-normal distribution of several variables including serum ferritin, nonparametric statistics were used for comparison between groups. Hardy–Weinberg equilibrium in genotype distribution was assessed with chi-square goodness-of-fit test. The 95% reference interval was defined as the 2.5–97.5 interpercentile range. Significance level was chosen at p < 0.05.
The association between HFE genotype and the risk of having elevated transferrin saturation and/or serum ferritin was assessed using conditional logistic regression to obtain adjusted odds ratios (OR) with 95% confidence interval (95%CI). The predicted phenotypic penetrances of HFE genotypes were calculated by the equation:
The probability of having elevated transferrin saturation or serum ferritin for a specific HFE genotype is an estimate of penetrance when other modifiers in the model are in the reference state (X i = 0). The reference group was wt/wt homozygous participants aged 32–39 years who never donated blood, had no self-reported disease, and had minimal alcohol, meat, milk, and egg consumption.
Results
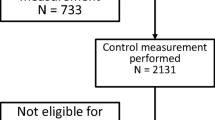
Among the 10,993 invited men, saliva samples were obtained from 6,567 out of 10,993 (60%) men. In 1,064 out of 6,567 (16%) men, the HFE genotype could not be assessed on saliva. They were invited for genetic testing and analyses of iron status markers; this was obtained in 522 out of 1,064 (49%) men of whom 331 out of 522 (60%) had HFE wild type. In total, HFE genotype was determined in 6,020 out of 10,993 (55%) men.
The 2,149 out of 6,020 (36%) men carrying a HFE variant were invited for analyses of iron status markers. Blood samples were drawn from 930 out of 2,149 (43%) men. Iron status markers were analyzed in 1,452 out of 6,020 (24%) men.
HFE allele frequencies
The HFE allele distributions were in Hardy–Weinberg equilibrium (χ 2 = 5.4, p = 0.8). In the entire series (n = 6,020), the allele frequency of C282Y, H63D, and S65C variants was 5.6%, 12.8%, and 1.8%, respectively. We found 1.4% C282Y/H63D, 0.1% C282Y/S65C, and 0.4% H63D/S65C compound heterozygotes [14].
Iron status markers
At blood sampling, 250 out of 1,452 (17%) men were nonfasting. Serum iron and transferrin saturation were significantly lower in fasting than in nonfasting men (Table 1). Consequently, we used only fasting serum iron values in the calculation of transferrin saturation and in the statistical analyses. There were no significant differences between fasting and nonfasting values of serum transferrin and serum ferritin, so these data were pooled.
HFE variants and iron status markers
Table 2 shows the association between HFE genotypes and iron status markers. C282Y and H63D homozygotes as well as compound heterozygotes had significantly higher iron status markers than wt/wt homozygotes. In contrast, S65C heterozygotes had significantly lower serum ferritin than wt/wt homozygotes.
Box plots of iron status markers in different HFE genotypes are displayed in Fig. 1, and box plots of serum ferritin and transferrin saturation illustrating the separate effects of C282Y and H63D genotypes in Fig. 2.
Table 3 and Fig. 3 show the impact of the various HFE genotypes on iron status markers. Among untreated C282Y homozygotes (n = 21) with iron status markers (transferrin saturation n = 18, serum ferritin n = 16), 89% had elevated transferrin saturation ≥50%, 94% had elevated serum ferritin ≥300 μg/L, and 88% had elevation of both iron status markers; seven out of 16 (44%) had serum ferritin values >800 μg/L (Table 6). Three C282Y homozygotes had normal iron status markers. Two were treated with phlebotomy. The third may have nonexpressivity of the C282Y variant, but repeat analysis of iron status markers was not available to make a reliable confirmation. Assuming that the two treated men initially had elevated iron status markers, the C282Y variant showed penetrance in 22 out of 23 men.
In addition, Table 3 displays the calculated penetrance, i.e., the effect of the HFE genotype on iron status markers, when other modifying factors in the model are in the reference state (see the “Statistics” section).
Logistic regression analysis
Tables 4 and 5 show the results of logistic regression used to estimate the risk of elevated transferrin saturation and serum ferritin associated with specific HFE genotypes. In C282Y homozygotes, the risk of elevated transferrin saturation was 86-fold higher and of elevated serum ferritin 66-fold higher than in wt/wt homozygotes. C282Y/H63D compound heterozygotes had a 7.2- and 3.3-fold higher risk of having elevated iron status markers than wt/wt homozygotes. Of the remaining genotypes, C282Y heterozygotes and H63D homozygotes had higher risk of having elevated transferrin saturation.
Table 6 shows details on the C282Y homozygotes. Iron status markers were available in 20 out of 23 men. One died during the study period, one moved to another district, and four did not respond to the follow-up invitation. Median age of the C282Y homozygotes at detection was 49 years.
Discussion
The present study assessed the penetrance of the three most frequent HFE variants on iron status markers. To our knowledge, this is the first study designed to perform primarily genetic screening for HFE hemochromatosis followed by secondary phenotypic evaluation. Consequently, the results of our study are not directly comparable with other penetrance studies. We decided that the screening population should consist of men who are more predisposed to develop iron overload than women, i.e., the influence of HFE variants is more likely to be expressed in men than in women [1, 12]. Women are partly protected against iron overload due to iron losses at menstruation and pregnancy [22]. In men, body iron reserves accumulate from adolescence to 30–35 years of age and subsequently remain at a stable level, which is individual and determined by genetic and environmental factors [23, 24]. In Danish patients with clinical hemochromatosis, the median age at diagnosis is ∼56 years [1]. Therefore, we chose to screen men 30 to 53 years of age. In order to evaluate a genetic effect, the population should be as homogeneous as possible, so we included men exclusively of Danish heritage.
In this series, the phenotypic penetrance in the 23 C282Y homozygous men was close to 100%, when the two phlebotomy-treated men were included in the estimates. Only one man had probable nonexpressivity. Unfortunately, we had no opportunity to evaluate the extent of iron-induced organ involvement in these men. The penetrance in C282Y/H63D compound heterozygotes was significant, but lower than in C282Y homozygotes.
HFE variant homozygous and compound heterozygous genotypes displayed significantly higher transferrin saturation than wt/wt homozygous genotype. Among wt/wt homozygotes, 5.7% had elevated transferrin saturation, 13.3% elevated serum ferritin, and 0.9% elevation of both iron status markers. These findings suggest that other genetic factors besides HFE variants play a role for body iron homeostasis.
We observed that the S65C variant had no significant influence on iron status markers compared with the C282Y and H63D variants (Table 3). S65C/wt heterozygotes actually had a lower frequency of elevated iron status markers than wt/wt heterozygotes. H63D/S65C compound heterozygotes had similar frequency of elevated iron status markers as wt/wt homozygotes but lower frequency than H63D/wt heterozygotes. Furthermore, C282Y/S63D compound heterozygotes had similar or lower frequency of elevated iron status markers than C282/wt heterozygotes. These results contrast an Australian study, which concluded that C282Y/S65C compound heterozygotes had an increased risk of phenotypic hemochromatosis [25] but is in accordance with a Norwegian study reporting that, although some C282Y/S65C compound heterozygotes have elevated iron status markers, the overall penetrance of this genotype is low [26]. Screening for S65C may be useful in individuals with iron overload who are not C282Y homozygous or C282Y/H63D compound heterozygous.
The majority of subjects of northern European descent with hemochromatosis have HFE hemochromatosis due to C282Y homozygosity [27]. The penetrance of C282Y homozygosity in various populations range from 0.7% [10] to 100% [4], depending on the criteria used. Most studies have included both genders despite differences in penetrance [4–10, 12]. Allen et al. [12] followed up 203 C282Y homozygotes during a 12-year period; 28% of men and 1% of women developed clinically overt iron overload. C282Y homozygotes with a serum ferritin level of >1,000 μg/L were at increased risk of hemochromatosis-associated signs and symptoms, when compared with either homozygotes with a serum ferritin level of ≤1,000 μg/L or individuals with other HFE genotypes [12].
The earliest phenotypic sign of HFE hemochromatosis is an elevated transferrin saturation [28]. The serum ferritin concentration reflects body iron accumulation over time and in the early stage of hemochromatosis serum ferritin is usually within normal range. We used a serum ferritin limit of 800 μg/L in the definition of major iron overload as we have experienced that nearly all such subjects have elevated serum transaminases [19].
The frequency of the C282Y allele in Denmark is ∼5.6%, corresponding to a homozygosity frequency of ∼0.31% [14]. According to calculations based on the National Census Registry in 2002 (population in Denmark ∼5,000,000) the estimated number of C282Y homozygotes in Denmark was ∼16,900, comprising ∼3,088 men aged 30–54 years.
In the logistic regression analysis, men with an expected higher risk had a significantly elevated calculated risk of having elevated iron status markers. In C282Y homozygotes, the OR for having elevated transferrin saturation was 86 and for having elevated serum ferritin it was 66 (Tables 4 and 5). In C282Y/H63D compound heterozygotes, the corresponding OR were 7.2 and 3.3, respectively.
The calculated penetrance of HFE genotypes on iron status markers was highest for C828Y homozygotes and C282Y/H63D compound heterozygotes (Table 3). However, the statistics should be interpreted with caution due to the small number of C282 homozygotes.
Previously, a diagnosis of hereditary hemochromatosis was based on the presence of clinical symptoms due to iron overload in combination with elevated iron status markers. In families with hemochromatosis, elevated iron status markers in parents/siblings to the probands were sufficient to make the diagnosis. In the screening situation, the diagnosis has so far been based on phenotypic expression of elevated iron status markers [28, 29].
With DNA-based technology, the diagnosis can be confirmed in the asymptomatic, preclinical stage of the disorder, and preventive measures can be initiated before organ damage occurs. HFE hemochromatosis has variable penetrance, and there is an ongoing discussion whether we should initiate screening programs in populations with high frequencies of HFE variants [12]. Although HFE hemochromatosis is the most prevalent genetic disorder in northern European populations, there have been raised doubts about whether it is appropriate to use genetic screening to diagnose preclinical cases [10].
HFE hemochromatosis fulfills the majority of the WHO criteria for population screening, yet several issues have to be more clarified before screening programs can be initiated, e.g., age- and gender-related penetrance of different HFE genotypes, interactions between HFE genotypes and environmental modifiers, and psychosocial consequences of screening [13, 30].
Serum iron displays significant 24-h and day-to-day variation and is influenced by meals. In order to reduce the number of high false-positive values, participants were told to be fasting at blood sampling [31]. Transferrin saturation has been widely used as initial screening procedure in HFE hemochromatosis with a cut-off value ranging from 45% to 60% [4–10, 27]. However, due to the variation in serum iron, transferrin saturation can be unreliable as demonstrated in some of our C282Y homozygotes (Table 6), which stresses the importance of repeated analyses of transferrin saturation when hemochromatosis is suspected. Serum ferritin is often included in the screening and there is consensus that the upper limit in men is ∼300 μg/L [21, 27, 32].
In conclusion, this study has combined initial genetic screening with subsequent phenotypic screening in HFE hemochromatosis. The C282Y variant is frequent in Danes and appears to have a high phenotypic penetrance in men, being close to 100%. From a cost-effective point of view, our results are in favor of screening Danish men above ∼40 years of age for hemochromatosis [33]. HFE hemochromatosis is a frequent disorder; phlebotomy treatment is cheap, effective, prevents organ damage, and ensures a normal lifespan and quality of life [1]. The potential psychological consequences of screening have raised much anxiety [30], which appears to be exaggerated. In Denmark, the population has a positive, practical, and cooperative attitude toward genetic screening surveys [13].
References
Milman N, Pedersen P, Steig T, Byg K-E, Graudal N, Fenger K (2001) Clinically overt hereditary hemochromatosis in Denmark 1948–1985: epidemiology, factors of significance for long-term survival, and causes of death in 179 patients. Ann Hematol 80:737–744 doi:10.1007/s002770100371
Milman N, Pedersen P (2003) Evidence that the Cys282Tyr mutation of the HFE gene originated from a population in Southern Scandinavia and spread with the Vikings. Clin Genet 64:36–47 doi:10.1034/j.1399-0004.2003.00083.x
Milman N (2000) Inheritance of hemochromatosis: family studies. In: Barton JC, Edwards CQ (eds) Hemochromatosis. Genetics, pathophysiology, diagnosis and treatment. Cambrigde University Press, Cambrigde, pp 15–41 ISBN 0 521 593808
Burt MJ, George PM, Upton JD, Collett JA, Frampton CM, Chapman TM, Walmsley TA, Chapman BA (1998) The significance of haemochromatosis gene mutations in the general population: implications for screening. Gut 43:830–836
Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW (1999) A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med 341:718–724 doi:10.1056/NEJM199909023411002
McDonnell SM, Hover A, Gloe D, Ou CY, Cogswell ME, Grummer-Strawn L (1999) Population-based screening for hemochromatosis using phenotypic and DNA testing among employees of health maintenance organizations in Springfield, Missouri. Am J Med 107:30–37 doi:10.1016/S0002-9343(99)00163-1
McLaren GD, McLaren CE, Adams PC, Barton JC, Reboussin DM, Gordeuk VR, Acton RT, Harris EL, Speechley MR, Sholinsky P, Dawkins FW, Snively BM, Vogt TM, Eckfeldt JH, Hemochromatosis and Iron Overload Screen (HEIRS) Study Research Investigators (2008) Clinical manifestations of hemochromatosis in HFE C282Y Homozygotes identified by screening. Can J Gastroenterol 22:923–930
Phatak PD, Ryan DH, Cappuccio J, Oakes D, Braggins C, Provenzano K, Eberly S, Sham RL (2002) Prevalence and penetrance of HFE mutations in 4865 unselected primary care patients. Blood Cells Mol Dis 29:41–47 doi:10.1006/bcmd.2002.0536
Jackson HA, Carter K, Darke C, Guttridge MG, Ravine D, Hutton RD, Napier JA, Worwood M (2001) HFE mutations, iron deficiency and overload in 10,500 blood donors. Br J Haematol 114:474–484 doi:10.1046/j.1365-2141.2001.02949.x
Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T (2002) Penetrance of 845G→A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet 359:211–218 doi:10.1016/S0140-6736(02)07447-0
Andersen RV, Tybjærg-Hansen A, Appleyard M, Birgens H, Nordestgaard BG (2004) Hemochromatosis mutations in the general population: iron overload progression rate. Blood 103:2914–2919 doi:10.1182/blood-2003-10-3564
Allen KJ, Gurrin LC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD, Anderson GJ, Southey MC, Giles GG, English DR, Hopper JL, Olynyk JK, Powell LW, Gertig DM (2008) Iron overload related disease in HFE hereditary hemochromatosis. N Engl J Med 358:221–230 doi:10.1056/NEJMoa073286
Elsass P, Pedersen P, Husum K, Milman N (2008) Assessment of the psychological effects of genetic screening of hereditary hemochromatosis. Ann Hematol 87:397–404 doi:10.1007/s00277-007-0415-2
Pedersen P, Melsen GV, Milman N (2008) Frequencies of the haemochromatosis (HFE) gene variants C828Y, H63D and S65C in 6,020 ethnic Danish men. Ann Hematol 87:735–740 doi:10.1007/s00277-008-0506-8
Rudbeck L, Dissing J (1998) Rapid, simple alkaline extraction of human genomic DNA from whole blood, buccal epithelial cells, semen and forensic stains for PCR. Biotechniques 25:588–592
Thorstensen K, Asberg A, Kvitland M, Svaasand E, Hveem K, Bjerve KS (2000) Detection of an unusual combination of mutations in the HFE gene for hemochromatosis. Genet Test 4:371–376 doi:10.1089/109065700750065117
Best LG, Harris PE, Spriggs EL (2001) Hemochromatosis mutations C282Y and H63D in ‘cis’ phase. Clin Genet 60:68–72 doi:10.1034/j.1399-0004.2001.600111.x
Lucotte G, Champenois T, Semonin O (2001) A rare case of a patient heterozygous for the hemochromatosis mutation C282Y and homozygous for H63D. Blood Cells Mol Dis 27:892–893 doi:10.1006/bcmd.2001.0451
Milman N (1991) Iron status markers in hereditary haemochromatosis: distinction between individuals being homozygous and heterozygous for the haemochromatosis allele. Eur J Haematol 47:292–298
Hamwi A, Endler G, Rubi K, Wagner O, Endler AT (2002) Reference values for a heterogeneous ferritin assay and traceability to the 3rd International Recombinant Standard for Ferritin (NIBSC code 94/572). Clin Chem Lab Med 40:365–370 doi:10.1515/CCLM.2002.059
Milman N (1996) Serum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy. Int J Hematol 63:103–135 doi:10.1016/0925-5710(95)00426-2
Milman N, Clausen J, Byg K-E (1998) Iron status in 268 Danish women aged 18–30 years: influence of menstruation, contraceptive method, and iron supplementation. Ann Hematol 77:13–19 doi:10.1007/s002770050405
Milman N, Ovesen L, Byg K-E, Graudal N (1999) Iron status in Danes updated 1994. I: prevalence of iron deficiency and iron overload in 1332 men aged 40–70 years. Influence of blood donation, alcohol intake, and iron supplementation. Ann Hematol 78:393–400 doi:10.1007/s002770050537
Milman N, Byg K-E, Ovesen L, Kirchhoff M, Jürgensen KS (2002) Iron status in Danish men 1984–94: a cohort comparison of changes in iron stores and the prevalence of iron deficiency and iron overload. Eur J Haematol 68:332–340 doi:10.1034/j.1600-0609.2002.01668.x
Wallace DF, Walker AP, Pietrangelo A, Clare M, Bomford AB, Dixon JL, Powell LW, Subramaniam VN, Dooley JS (2002) Frequency of the S65C mutation of HFE and iron overload in 309 subjects heterozygous for C282Y. J Hepatol 36:474–479 doi:10.1016/S0168-8278(01)00304-X
Asberg A, Thorstensen K, Hveem K, Bjerve KS (2002) Hereditary hemochromatosis: the clinical significance of the S65C mutation. Genet Test 6:59–62 doi:10.1089/109065702760093933
Milman N, Pedersen P, Steig T, Melsen GV (2003) Frequencies of the hereditary hemochromatosis allele in different populations. Comparison of previous phenotypic methods and novel genotypic methods. Int J Hematol 7:48–54 doi:10.1007/BF02982602
Edwards CQ, Kushner JP (1993) Screening for hemochromatosis. N Engl J Med 328:1616–1620 doi:10.1056/NEJM199306033282208
George DK, Powell LW (1997) Review article: the screening, diagnosis and optimal management of haemochromatosis. Aliment Pharmacol Ther 11:631–639 doi:10.1046/j.1365-2036.1997.00197.x
Adams P, Brissot P, Powell LW (2000) EASL International Consensus Conference on Haemochromatosis. J Hepatol 33:485–504 doi:10.1016/S0168-8278(01)80874-6
Edwards CQ, Griffen LM, Kaplan J, Kushner JP (1989) Twenty-four hour variation of transferrin saturation in treated and untreated haemochromatosis homozygotes. J Intern Med 226:373–379
Munro HN, Linder MC (1978) Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev 58:317–396
Allen KJ (2008) Population genetic screening for hereditary haemochromatosis: are we a step closer? Med J Aust 189:300–301
Acknowledgements
The authors express their gratitude to the medical staff and laboratory technicians at the Department of Clinical Biochemistry at Næstved Hospital for their enthusiasm and great effort in conducting this study. Thanks to Chief Physician Arne Bremmelgaard for the encouragement and excellent guidance and to Laboratory Technician Gitte Vedel Melsen for the skillfull technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pedersen, P., Milman, N. Genetic screening for HFE hemochromatosis in 6,020 Danish men: penetrance of C282Y, H63D, and S65C variants. Ann Hematol 88, 775–784 (2009). https://doi.org/10.1007/s00277-008-0679-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-008-0679-1