Abstract
We investigated the concomitant interim response of patients with aggressive non-Hodgkin’s lymphoma (NHL) using multi-detector row computerized tomography (CT) and 18F-fluoro-2-deoxy-d-glucose-positron emission tomography (PET)/CT for prediction of clinical outcomes. One hundred six newly diagnosed patients with aggressive NHL were enrolled. Both the CT and PET/CT were serially performed at the time of diagnosis and after three to four cycles of chemotherapy (interim). The patients were categorized into four different responsive groups according to the interim PET/CT and CT: (1) complete metabolic response (CMR)–complete response unconfirmed (CRu), (2) CMR–partial response (PR), (3) partial metabolic response (PMR)–Cru, and (4) PMR–PR. Fifty-five patients with CMR–CRu, 20 patients with CMR–PR, seven patients with PMR–Cru, and 23 patients with PMR–PR were distributed. In addition, one patient experienced a disease progression. There was a significant difference in relapse rates between PET/CT-positive (67.3%) and PET/CT-negative patients (17.3%; P < 0.01). Also, there was a significant difference between patients with PMR–PR (32.0% and 26.1%) and CMR–CRu (89.3% and 80.0%) for 3-year overall survival (OS) and event-free survival (EFS), respectively. A multivariate analysis revealed that high international prognostic index (≥3) at diagnosis, T-cell phenotype, and PMR–PR in interim PET/CT and CT were independent prognostic significances for OS. Moreover, bulky disease (>10 cm), T-cell phenotype, and PMR–PR showed significant associations for EFS. PMR–PR in interim response was the predictive prognostic determinant for both OS and EFS, with a hazard ratio of 3.93 (1.61–9.60) and 3.60 (1.62–7.98), respectively. The combined evaluation of interim PET/CT and CT was found to be a significant predictor of disease progression, OS, and EFS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Whole-body positron emission tomography (PET) with 18F-fluoro-2-deoxy-d-glucose (FDG) is a functional imaging modality used as a staging and monitoring response for the treatment of malignant lymphoma, which, in turn, has a higher sensitivity and specificity than conventional imaging. The residual abnormalities following chemotherapy, representing the development of fibrosis or tumor necrosis, are seen in up to 64% of lymphoma patients [1–3]. Conventional imaging, especially multi-detector row computerized tomography (CT), cannot reliably help in the differentiation between active tumors and fibrosis or necrosis [4–7]. The limitations of conventional CT have restricted the predictive value of conventional CT to the clinical outcome of non-Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma. Nevertheless, of these limitations, conventional CT is still easily used for evaluating the therapeutic response during or after chemotherapy in malignant lymphoma.
PET/CT may be a more accurate tool for assessing treatment effects, correctly identifying patient with residual disease, and predicting therapeutic outcomes than conventional imaging. Further, FDG uptake is a tool used to predict the therapeutic response during or after the course of treatment. Several studies have demonstrated the prognostic value of post-therapeutic FDG-PET or PET/CT in malignant lymphoma [3, 8–10]. Moreover, higher relapse rates and less event-free survival (EFS) have been observed in PET-positive than in PET-negative patients [11–13]. FDG-PET or PET/CT images can predict the increased risk of treatment failure during or after primary chemotherapy. However, since FDG is not a tumor-specific substance, it may accumulate to the point of being detected in a variety of benign conditions, which may give rise to false-positive results. A correlation with findings of anatomic imaging such as CT is an important for identifying changes resulting from chemotherapy.
In this study, we investigated the concomitant interim response of patients with aggressive NHL using conventional CT and PET/CT as a possible predictor of the clinical outcome prior to completing of primary chemotherapy. We also determined the differentiation potentials to provide a risk-adapted therapeutic strategy for patients with aggressive NHL.
Materials and methods
Patients and study design
One hundred six newly diagnosed patients with aggressive NHL were enrolled between August 2004 and June 2008 at Chonnam National University Hwasun Hospital. All patients were subjected to the interim response analysis, both CT and PET/CT, after obtaining their informed consent. We excluded the patients with early disease progression (after first or second cycle) or personal disagreement. All patients were initially performed a CT and PET/CT at diagnosis with a subsequent follow-up interim CT and PET/CT after the third or fourth primary chemotherapy. The interim response evaluation of CT and PET/CT was performed a day prior to a scheduled chemotherapy. The final response was assessed within a month of completing chemotherapy, with follow-up restaging every 3 months during the first year after chemotherapy and every 6 months thereafter. Patients with localized lymphoma (stage I) were treated with three or four cycles of chemotherapy followed by involved field radiation therapy (IFRT). Alternatively, patients with advanced stage were treated with eight cycles of chemotherapy. However, patients aged greater than 60 were treated with six cycles of primary chemotherapy if they achieved a complete response (CR) for the interim PET/CT. Finally, patients with an initial tumor size larger than 10 cm or with mediastinal disease exceeding one third were categorized as having bulky disease.
Treatment protocol
Patients with diffuse large B cells received R-CHOP (rituximab 375 mg/m2 i.v. on day 1 (D1), cyclophosphamide 750 mg/m2 i.v. on D1, vincristine 1.4 mg/m2 i.v. on D1, doxorubicin 50 mg/m2 i.v. on D1, and prednisolone 100 mg p.o. on D1–5) in standard doses every 3 weeks, and those with peripheral T-cell lymphoma (PTCL) and natural killer/T-cell lymphoma received CHOP-E (etoposide 100 mg/m2 i.v. on D1–2) or Alem-CHOP (alemtuzumab 30 mg i.v. on D1) every 3 weeks. Patients with localized disease were given IFRT (30 Gy) after third or fourth cycle of chemotherapy.
Response evaluation
The initial-staging CT and the interim CT were assessed according to International Workshop Criteria [4]. PET/CT imaging was analyzed according to the combination of morphology by the CT portion (the size and shape of nodes and the number of remaining nodes) and the metabolic uptake by the FDG-PET portion. We classified patients based on three mid-response criteria of PET/CT using the semi-quantitative assessment of the maximal standardized uptake value (SUVmax; Table 1).
Statistics
All statistical analyses were performed using SPSS statistical software version 12.0 (SPSS, Chicago, IL, USA). Event-free survival was calculated from the treatment start time to the first recording of disease progression or death from any cause. Patients whose disease did not progress would be censored using the date at which they were last known to show no progress. Overall survival (OS) was defined as the period from treatment start time to the date of last follow-up or death from any cause. Patients who were subjected to high-dose chemotherapy followed by autologous stem cell transplantation (ASCT), would be censored at the time of transplantation. The distribution of patient for OS and EFS was estimated using the method of Kaplan–Meier and were compared by the log-rank test for the association between clinical prognostic factors and the probability of treatment failure. Multivariate Cox’s proportional-hazards models were used to analyze all the influences which were found to be significant in the univariate analysis. P values <0.05 were considered statistically significant, and the results were expressed as the mean ± SEM.
Results
The patient’s clinical characteristics are summarized in Table 2. In brief, the median age of patients was 59 years (range, 17–85 years) with 46.2% of patients aged above 60. Ninety (84.9%) of the enrolled patients had diffuse large B cell lymphoma (DLBCL), and 17 (16.0%) had bulky disease. The median interval from the moment of diagnosis to interim response time was 95 days (range, 34–281 days) and the median follow-up duration 24.3 months (range, 5.8–52.9 months). Over the course of the follow-up period, 34 (32.1%) patients experienced a relapse, 12 (11.3%) underwent high dose chemotherapy followed by ASCT, and 27 (25.5%) were censored due to death.
Sixty-two (58.5%) patients achieved unconfirmed CR (CRu), and 44 (41.5 %) showed partial response (PR) by interim CT. Alternatively, 75 (70.8%) patients achieved complete metabolic response (CMR), and 1 (0.9%) showed no metabolic response (NMR) by interim PET/CT. Upon analysis of the therapeutic response after primary chemotherapy, 87 (82.1%) patients achieved CR, 10 (9.4%) achieved PR, one (0.9%) showed stable disease (SD), and eight (7.5%) showed disease progression. For the interim CT analysis, 11 (17.7%) and 23 (52.3%) patients who have achieved CRu and PR, respectively, experienced a relapse (P < 0.01). In comparing the interim PET/CT with the final CT response after primary chemotherapy, 70 (93.3%) of 75 patients who achieved CMR in interim PET/CT concordantly maintained a complete response at the completion of primary chemotherapy, whereas 13 (17.3%) patients relapsed after the primary chemotherapy (Table 3). The patients with positive PET/CT showed an extremely higher relapse rate (67.7%) regardless of interim CT response (P < 0.01).
The response group categorized according to the combination of interim PET/CT and CT response (55 patients with CMR–CRu, 20 patients with CMR–PR, seven patients with PMR–CRu, and 23 patients with PMR–PR) had relapse rates of 14.5% for the CMR–CRu, 25.0% for the CMR–PR, 42.9% for the PMR–Cru, and 73.9% for the PMR–PR group during or after primary chemotherapy (P < 0.01).
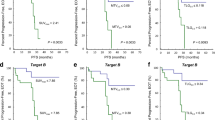
The 3-year probability of OS and EFS was 72.1% and 66.4%, respectively. In univariate analysis, histological subtype (diffuse large B cell vs peripheral T cell), stage, bulky disease, and international prognostic index (IPI) were significant prognostic variables for OS and EFS. In addition, both the interim CT and PET/CT response showed a significant potential as prognostic variable in OS and EFS. However, the interim PET/CT showed high differentiation potency in predicting the prognosis for OS and EFS (Fig. 1). When we analyzed the clinical outcome based on histologic subtypes, patients with T-cell lymphoma had a poor prognosis for OS and EFS compared to those with DLBCL. Interim PET/CT in T-cell lymphoma, even though their limited number of enrolled patients, had a less potential as prognostic determinant than in DLBCL (Fig. 2). We also found significant differences between the patients with PMR–PR (32.0% and 26.1%) and the patients with CMR–CRu (89.3% and 80.0%) in 3-year OS and EFS, respectively. We identified that the patients with PMR–CRu (35.7%) had an extremely worse prognosis than patients with CMR–PR (75.0%) in the 3-year EFS (Fig. 2).
Following a multivariate analysis, high IPI (≥3) at diagnosis, T-cell phenotype, and PMR–PR in interim PET/CT and CT showed independent prognostic variables for OS. Bulky lesion at diagnosis, T-cell phenotype, and PMR–PR in interim PET/CT and CT emerged that provided significantly independent prognosis of EFS (Table 4). PMR–PR in interim response was the predictive prognostic determinant for both OS and EFS, with a hazard ratio of 3.93 (1.61–9.60) and 3.60 (1.62–7.98), respectively
Two patients determined to have positive uptake by interim PET/CT revealed false positives after primary chemotherapy. One patient maintained significant metabolic uptakes in the mediastinal lymph nodes, which were confirmed to be consequences of tuberculous lymphadenitis by a bronchoscopic biopsy, whereas the other patient showed new lesions in the brain parenchyma and was diagnosed with astrocytoma via a stereotactic biopsy. On the other hand, one patient with cutaneous T-cell lymphoma had a false negative for the interim PET/CT, nevertheless partially remaining skin lesions.
Discussion
Patients with aggressive NHL are stratified into prognostic groups based on the IPI and molecular profiling of diffuse large B cell lymphoma [14, 15]. These therapeutic measures could make it possible to predict the survival after chemotherapy as well as to alter the therapeutic strategies to poor-risk groups. Recently, the addition of anti-CD20 monoclonal antibody to CHOP (R-CHOP) has improved the therapeutic outcomes and changed the survival of prognostic groups using the IPI [16]. However, the risk stratification is usually based on the prognostic characteristics of IPI, which does not predict the individual response to primary chemotherapy or intensive chemotherapy.
The early treatment response assessed by CT may be helpful in predicting the clinical outcomes [17]; however, conventional CT could not differentiate between viable tumor tissue and necrotic residual scarring. Moreover, several of partially responsive patients, assessed from interim CT, may include the patients with residual scar tissue who achieved a complete response after primary chemotherapy [18]. In our investigation, the interim CT analysis mirrored the clinical results which demonstrated that early complete responsive patients had a better prognosis in OS and EFS than the partial responsive patients; although interim, the CT response did not match the final treatment response. However, the patients who achieved CMR by interim PET/CT maintained the clinical response until the completion of primary chemotherapy. Moreover, 91.9% of patients with CMR matched the patients with complete response in the final response analysis.
Recent studies demonstrated that the prognostic value of FDG-PET, shortly after onset of induction chemotherapy or at mid-treatment, could predict the long-term clinical outcome in patients with Hodgkin’s disease or NHL [10, 19–22]. These studies categorized patients via the PET-positive and PET-negative analysis and subsequently compared the relapse rate and the progression/failure-free survival between the two groups. The relapse rates in PET-positive patients were 70–80% and consequently had a lower EFS and OS. Moreover, to predict the progression/event-free survival or the OS, the interim FDG-PET or PET/CT was superior to conventional CT. However, the tumor aggressiveness could affect the FDG uptakes after the early or mid-treatment response to chemotherapy, as a result of FDG avidity in differentiating between indolent and aggressive lymphomas [23, 24].
Reinhardt et al. [25] reported that the combined evaluation of post-therapeutic CT and FDG-PET showed a more differentiated assessment of individual prognosis in lymphoma patients. This is particularly true in patients with PR and SD for CT and could be differentiated according to post-therapeutic FDG-PET. In this study, we evaluated the aggressive NHL patients using a combined assessment approach by looking at results from interim CT and PET/CT, which render it possible to make a tailored therapeutic plan after primary chemotherapy in patients with a poor prognosis. Our results are consistent with those of previous studies stating that the patients with CMR–CRu and CMR–PR have a better prognosis than the patients with PMR–CRu and PMR–PR in OS and EFS. After analyzing the patients who achieved PMR in detail, the relapse rates between PMR–CRu (33.3%) and PMR–PR (70.0%) were significantly different. Moreover, patients with PMR–PR had a significant worse prognosis than patients with PMR–CRu for the 2-year OS (Fig. 2). A multivariate analysis found that the combined evaluation of positivity and size of tumor mass, as a function of interim PET/CT and conventional CT, was independent prognostic factor and had a stronger predictive value than all other factors (Fig. 3).
The prognostic significance of early PET scans after first or second chemotherapy may be result in a false determination due to the tracer uptakes of inflammatory or infectious lesions [26]. Schöder and Moskowitz in Sloan-Kettering Cancer Center recently suggested the reasons for false-positive and false-negative findings at the end of therapy or an interim PET scan [27]. The physiologic variants, such as intense uptake in brown adipose tissue or skeletal muscles, the intensity of normal FDG uptake in gastrointestinal tracts, the nonspecific uptake in normal-sized or mildly enlarged inguinal nodes, and the new foci of uptake in the lungs in a patient showing otherwise good response to therapy may cause high false-positive findings in PET scan. They suggest that it is mandatory to confirm suspicious FDG uptake with biopsy if scan findings are to be used to alter therapy at interim. We also experienced a discrepancy between CT staging and PET/CT staging at diagnosis. Three cases of gastrointestinal positive in PET/CT without abnormal findings in contrast-enhanced CT revealed the physiologic uptakes by duodenoscopy or colonoscopy, and one case of pulmonary positive finding revealed the benign inflammatory nodule. However, the sequential PET/CT and CT may decrease the false positive or negative predictive value by the assessment of SUV changes compared to the initial FDG uptakes and locations and the assessment of the anatomic findings. We observed two cases of patients with false positive in the interim PET/CT due to the infection and the newly developed, other malignancy.
In conclusion, the clinical assessment after the third or fourth chemotherapy by conventional CT and PET/CT provided significant predictive value for disease progression, OS, and EFS. The patients who achieved the PMR and PR in interim PET/CT and CT should be considered for intensive therapeutic plans, including high-dose chemotherapy with SCT.
References
Spaepen K, Stroobants S, Dupont P, Van Steenweghen S, Thomas J, Vandenberghe P et al (2001) Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol 19:414–419
Jerusalem G, Beguin Y, Fassotte MF, Najjar F, Paulus P, Rigo P et al (1999) Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin’s disease and non-Hodgkin’s lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood 94:429–433
Mikhaeel NG, Timothy AR, O’Doherty MJ, Hain S, Maisey MN (2000) 18-FDG-PET as a prognostic indicator in the treatment of aggressive non-Hodgkin’s lymphoma—comparison with CT. Leuk Lymphoma 39:543–553
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al (1999) Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 17:1244
Surbone A, Longo DL, DeVita VT Jr, Ihde DC, Duffey PL, Jaffe ES et al (1988) Residual abdominal masses in aggressive non-Hodgkin’s lymphoma after combination chemotherapy: significance and management. J Clin Oncol 6:1832–1837
Coiffier B, Gisselbrecht C, Herbrecht R, Tilly H, Bosly A, Brousse N (1989) LNH-84 regimen: a multicenter study of intensive chemotherapy in 737 patients with aggressive malignant lymphoma. J Clin Oncol 7:1018–1026
Canellos GP (1988) Residual mass in lymphoma may not be residual disease. J Clin Oncol 6:931–933
Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ (2002) PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin’s disease. J Nucl Med 43:1018–1027
Kostakoglu L (2002) Noninvasive detection of multidrug resistance in patients with hematological malignancies: are we there yet? Clin Lymphoma 2:242–248
Querellou S, Valette F, Bodet-Milin C, Oudoux A, Carlier T, Harousseau JL et al (2006) FDG-PET/CT predicts outcome in patients with aggressive non-Hodgkin’s lymphoma and Hodgkin’s disease. Ann Hematol 85:759–767 doi:10.1007/s00277-006-0151-z
Picardi M, De Renzo A, Pane F, Nicolai E, Pacelli R, Salvatore M et al (2007) Randomized comparison of consolidation radiation versus observation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma 48:1721–1727 doi:10.1080/10428190701559140
Zhao J, Qiao W, Wang C, Wang T, Xing Y (2007) Therapeutic evaluation and prognostic value of interim hybrid PET/CT with (18)F-FDG after three to four cycles of chemotherapy in non-Hodgkin’s lymphoma. Hematology 12(5):423–430
Fields PA, Mikhaeel G, Hutchings M, van der Walt J, Nunan T, Schey SA (2005) The prognostic value of interim positron emission tomography scans combined with immunohistochemical data in diffuse large B-cell lymphoma. Haematologica 90:1711–1713
(1993) A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 329:987–994. doi:10.1056/NEJM199309303291402
Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI et al (2002) The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937–1947 doi:10.1056/NEJMoa012914
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P et al (2007) The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109:1857–1861 doi:10.1182/blood-2006-08-038257
Armitage JO, Weisenburger DD, Hutchins M, Moravec DF, Dowling M, Sorensen S et al (1986) Chemotherapy for diffuse large-cell lymphoma—rapidly responding patients have more durable remissions. J Clin Oncol 4:160–164
Rodriguez-Catarino M, Jerkeman M, Ahlstrom H, Glimelius B, Hagberg H (2000) Residual mass in aggressive lymphoma–does size, measured by computed tomography, influence clinical outcome? Acta Oncol 39:485–489 doi:10.1080/028418600750013393
Haioun C, Itti E, Rahmouni A, Brice P, Rain JD, Belhadj K et al (2005) [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood 106:1376–1381 doi:10.1182/blood-2005-01-0272
Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR (2005) FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol 16:1514–1523 doi:10.1093/annonc/mdi272
Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR (2005) Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol 16:1160–1168 doi:10.1093/annonc/mdi200
Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J et al (2006) FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 107:52–59 doi:10.1182/blood-2005-06-2252
Lapela M, Leskinen S, Minn HR, Lindholm P, Klemi PJ, Soderstrom KO et al (1995) Increased glucose metabolism in untreated non-Hodgkin’s lymphoma: a study with positron emission tomography and fluorine-18-fluorodeoxyglucose. Blood 86:3522–3527
Schoder H, Noy A, Gonen M, Weng L, Green D, Erdi YE et al (2005) Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol 23:4643–4651 doi:10.1200/JCO.2005.12.072
Reinhardt MJ, Herkel C, Altehoefer C, Finke J, Moser E (2005) Computed tomography and 18F-FDG positron emission tomography for therapy control of Hodgkin’s and non-Hodgkin’s lymphoma patients: when do we really need FDG-PET? Ann Oncol 16:1524–1529 doi:10.1093/annonc/mdi271
Castellucci P, Nanni C, Farsad M, Alinari L, Zinzani P, Stefoni V et al (2005) Potential pitfalls of 18F-FDG PET in a large series of patients treated for malignant lymphoma: prevalence and scan interpretation. Nucl Med Commun 26:689–694 doi:10.1097/01.mnm.0000171781.11027.bb
Schoder H, Moskowitz C (2008) PET imaging for response assessment in lymphoma: potential and limitations. Radiol Clin North Am 46:225–241 doi:10.1016/j.rcl.2008.04.002
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, DH., Min, JJ., Jeong, Y.Y. et al. The combined evaluation of interim contrast-enhanced computerized tomography (CT) and FDG-PET/CT predicts the clinical outcomes and may impact on the therapeutic plans in patients with aggressive non-Hodgkin’s lymphoma. Ann Hematol 88, 425–432 (2009). https://doi.org/10.1007/s00277-008-0616-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-008-0616-3