Abstract
Although consensus exists relating criteria for the identification of low-risk patients with febrile neutropenia, no clear indication on how to manage these patients has been so far provided particularly in outpatients affected by hematologic malignancies. The feasibility and safety of early discharge was prospectively evaluated in 100 outpatients with hematologic malignancies and febrile neutropenia. A strategy considering the risk-index of the Multinational Association of Supportive Care in Cancer (MASCC) was applied. High-risk patients were entirely managed at hospital. Low-risk patients were early discharged if they were afebrile since 48 h and not on supportive therapy requiring hospitalization. Out of 90 low-risk episodes, in 69 instances (76.7%), patients were discharged after a median of 4 days and continued home therapy with oral cefixime (78%) or other antibiotics. Only five outpatients (7.2%) had fever recurrence. Twenty-one low-risk patients were not early discharged due to worsening conditions (three deaths), need of multiple daily dose therapy, or discharge refuse. No clinical characteristic was able to predict the eligibility for early discharge. The MASCC risk-index is a useful aid in the identification of high-risk febrile neutropenia needing whole in-hospital treatment. As for low-risk patients, hospitalization at least in the first days of fever is required. Cefixime could be included among the oral antibacterial drugs to be used in the outpatient treatment of adult patients with febrile neutropenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical approach to febrile patients with neutropenia has been a challenge in the years with the development of both new antimicrobial drugs and new strategies for using them. Over the past decade, with a better understanding of the syndrome of febrile neutropenia, it has become possible to categorize patients into risk-groups and to predict the outcome of this complication [1]. Various risk models based on patient characteristics at the onset of febrile neutropenia have been developed enabling clinicians to classify patients as low or high risk [1–5]. In 2000, the Multinational Association of Supportive Care of Cancer (MASCC) developed a prediction model aimed at identifying those patients with the greatest chance of recovering without serious medical complications [5]. Prognostic factors included in this model are listed in Table 1. Although consensus exists relating criteria to be considered for the identification of low-risk patients with febrile neutropenia, no clear indication on how to manage these patients has been so far provided, and different options include outpatient treatment with oral antibiotics or early discharge after in-hospital clinical evaluation in the first phase of the febrile episode [1, 4, 6–22].
In recent years, it has been a common practice in our center to manage a large number of patients with hematological malignancies into an outpatient setting even during the periods of severe neutropenia [5, 16, 23–25]. In the event of a febrile episode, patients were instructed to return immediately to our hematologic emergency unit (EU), which is specifically dedicated to outpatients with blood disorders requiring prompt clinical and therapeutic intervention for all kinds of hematologic emergencies [24, 25]. After an initial hospitalization phase, patients free from fever and in good clinical condition were usually discharged regardless of their absolute neutrophil and platelet count and continued antibiotic therapy as outpatients. To better assess the safety and efficacy of this early discharge approach, we prospectively evaluated 100 consecutive episodes of fever and neutropenia seen during an 18-month period. A risk-based strategy, which considered the MASCC risk-index, was applied to identify objective criteria for the choice of early hospital discharge of patients.
Materials and methods
Patients were from the population of adult subjects (>16 years) with hematological malignancies followed at the Dipartimento di Biotecnologie Cellulari ed Ematologia of the University “La Sapienza” of Rome, who referred to the hematologic EU and were hospitalized for febrile neutropenia from March 2001 through August 2002. Neutropenia was defined as an absolute neutrophil count of <500 cells/μl of blood and fever as a single body temperature >38.5°C or a temperature >38°C for more than 1 h. Upon arrival at the EU, a complete clinical and laboratory check was performed: medical history, physical examination, routine hematochemical analyses, three blood cultures as well as cultures from any presumed site of infection, and a chest radiograph. The MASCC risk-index score was calculated according to criteria shown in Table 1 for each patient. Patients with a score of ≥21 were regarded as low risk of complications, whereas patients with a score <21 were regarded as high risk. Within 1 h of arrival at the EU, patients with febrile neutropenia were admitted to the ward and empirically treated with the antibiotic intravenous combination ceftriaxone (2 g/24 h) plus amikacin (20 mg/kg/24 h). Patients with persisting fever after 4 days antibacterial therapy underwent chest CT scan and further microbiological exams. Therapy was adjusted on the basis of clinical response, results of culture, and sensitivity tests. In the event of persisting fever of unknown origin, usually teicoplanin was empirically added after 4 days and antifungal treatment after 7 days. The therapeutic plan was to continue antibiotics until 6 consecutive days has passed without fever or until microbiological and/or clinical evidence of infection disappeared.
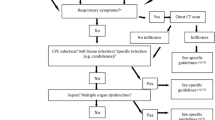
High-risk patients (score < 21) were managed at hospital for the entire therapeutic course regardless of neutropenia recovery and response to treatment. Low-risk patients (score ≥ 21) were early discharged to continue antibiotic therapy as outpatients, regardless of their absolute neutrophil or platelet counts, if they were free from fever since 48 h, were in good general conditions, and not on supportive therapy requiring further hospitalization. The decision for early discharge of each patient was made by the one responsible for clinical care with the informed consent of the patient. Patients eligible for early discharge were given oral cefixime (one 400 mg capsule every 24 h) for further 4 days if the initial antibiotic therapy with ceftriaxone plus amikacin had not been modified, the episode of febrile neutropenia was of unknown origin, or a microorganism susceptible to cefixime had been isolated from cultures. Patients eligible for early discharge but had initial antibiotic therapy modified, or a documented pulmonary infiltrate or a microbiologically documented infection by a pathogen not susceptible to cefixime, were given a different, adapted, parenteral, or oral antibiotic therapy. After discharge, patients were followed on an outpatient setting to monitor the outcome. The study schema is summarized in Fig. 1.
Our protocol was approved before the start of the study by a committee on human experimentation of our Institution, and informed consent was obtained from all our patients.
Results
During the 18-month study period, 100 consecutive episodes of febrile neutropenia in 87 outpatients requiring hospitalization were considered. The patient characteristics are summarized in Table 2. In three fourths of the episodes, patients had recently received remission induction chemotherapy or relapse treatment of underlying disease, whereas about 20% of the episodes occurred in patients who were on supportive/conservative therapy. Overall duration of neutropenia [absolute neutrophil count (ANC) < 500/μl] was mean 12.3 days (range, 2–104 days). In 60% of episodes, patients had neutropenia lasting >10 days. More than 50% of patients were profoundly neutropenic (ANC < 100/μl) and under oral ciprofloxacin prophylaxis (500 mg twice daily) at the start of fever. The clinical and microbiological characteristics of the 100 febrile episodes categorized according to the MASCC risk-index score are detailed in Table 3. At the time of hospital admission, 90 of 100 febrile episodes were associated with low risk (score ≥ 21), whereas the remaining ten episodes were at high risk (score < 21). The weight of the single prognostic factors used for score calculation was detailed. The factors which mainly affected the risk were age, hydration status, and burden of illness. Overall, 68%, 15%, and 17% of the febrile episodes were of unknown origin, clinically documented and microbiologically documented, respectively. Pneumonia was documented in 16 cases (2 of which with septicemia). In all but one microbiologically documented infection, the pathogen was isolated from blood (Table 4). Overall, 11 of 17 microbiologically documented infections occurred while the patients were under ciprofloxacin prophylaxis since 5 mean days (range, 2–9 days).
The initial empiric antibacterial therapy with ceftriaxone plus amikacin was maintained during hospitalization in 94% of cases. Initial antibiotic therapy was modified in six cases, as detailed in Table 3, based on microbiological data (Pseudomonas aeruginosa septicemia, two cases; Escherichia coli and Staphylococcus septicemia, one case each) or empirically (two cases). No antifungal treatment was required. Median duration of neutropenia from start of antibiotic therapy to achievement of ANC > 500/μl was 7 days (range, 2–14 days). Treatment success with initial antibacterial therapy or after modification was obtained in 97% of cases. Fever disappeared after a median of 3 days (range, 1–9 days). Seven (three high-risk and four low-risk) patients died, and in three of them (all at high-risk), death was related to the infectious episode (pneumonia in one patient and gram-negative septicemia in two patients).
The data of the clinical trial profile are detailed in Fig. 2. In 69 (76.7%) out of 90 episodes of febrile neutropenia at low risk (score ≥ 21), patients were early discharged after a median of 4 days (range, 2–6 days) before completion of the antibiotic therapy and continued antibacterial therapy at home. In 54 of the 69 cases (78%), antibiotic therapy was changed to oral cefixime, and in 15 cases not eligible for treatment with cefixime, patients continued the same parenteral antibacterial therapy (14 cases) or changed to oral levofloxacin (1 case) according to clinical or microbiological indications. Out of the 69 patients early discharged to continue antibacterial therapy on outpatient setting, 38 (55%) were still neutropenic the day of discharge and neutropenia lasted median 3 days (range, 1–10 days). Only five (7.2%) patients had fever recurrence during outpatient therapy or within 5 days from treatment discontinuation, with two of these patients requiring re-hospitalization. In 31 episodes of febrile neutropenia, the patients were hospitalized until completion of the antibiotic treatment or until death (median hospitalization time, 9 days; range, 2–30 days); ten patients were at high risk (score < 21), thus were considered not eligible for early discharge by protocol (four patients died). The remaining 21 febrile episodes occurred in patients at low risk (median MASCC score, 24; range, 21–26) who were not early discharged for the following reasons: worsening of clinical conditions related to the infectious complication or to the underlying hematological disease (15 cases, 3 of which died), need of multiple daily dose antibiotic therapy (2 cases), and refuse of early discharge before completion of intravenous therapy (4 cases). Considering the 90 febrile episodes at low risk, we compared the 69 episodes early discharged with the 21 who required prolonged hospitalization. No statistically significant difference was observed as to MASCC scoring system characteristics, type of febrile episodes, and duration of neutropenia after onset of fever (Table 5).
In the 69 episodes with early discharge, hospitalization lasted 306 cumulative days (median, 4 days; range, 2–6 days), and antibiotic therapy continued out of the hospital for 282 cumulative days (median, 4 days; range, 2–14 days). In the 31 episodes not eligible for early discharge, patients were hospitalized for 280 cumulative days (median, 7 days; range, 2–30 days).
Discussion
The model developed by the MASCC is now considered a standard system to identify febrile neutropenic patients at different risk for serious complications, including mortality [5]. This model represents an improvement over the previous classification validated by Talcott et al. [3] in that it has a substantially increased rate of identification of patients as being at low risk, with a positive predictive value increasing with the increase of the threshold to define the clinical prediction rule [5]. A major problem in the definition of risk for serious complications in a patient with febrile neutropenia is represented by choice of a personalized management. Although hospitalization and close clinical monitoring appear necessary in all patients with high-risk febrile neutropenia, the clinical approach to low-risk patients has not yet been standardized. The 2002 guidelines of the Infectious Diseases Society of America underline that the use of oral antibiotics may be considered for low-risk patients, provided that a vigilant observation and prompt access to appropriate medical care is ensured 24 h per day, 7 days per week [26]. As a matter of fact, the whole management of a febrile neutropenia on an outpatient setting cannot be recommended, but an initial inpatient intravenous therapy, with fulminant infection exclusion and culture specimens ascertainment, seems to be a safer approach [21, 22]. This caution seems particularly justified for patients with hematological malignancies. Furthermore, it should be also considered that the MASCC study related to a general population of cancer patients with different clinical characteristics and only a minority of patients with active hematologic malignancies who typically develop prolonged pancytopenia requiring careful clinical and therapeutic support.
To date, only few experiences have been reported relating to prospective clinical application of the MASCC risk-index score [27, 28], and a single study [28] focused on management of febrile neutropenia in patients with hematological malignancies. In this study, 279 episodes of febrile neutropenia were prospectively included and the feasibility and safety of early discharge after fever defervescence with subsequent oral antibiotic therapy was evaluated in those patients with a MASCC low-risk score (38% of the total episodes). A significant difference in the occurrence of serious complications was observed according to risk; however, over one third of the low-risk patients were considered ineligible for early discharge and oral therapy.
Our experience substantially confirms the results of this study [28]. In fact, the clinical evolution and the mortality rate of the few patients with high-risk score as evaluated at the time of hospital admission, confirm the need of a careful in-hospital management in this category of patients. On the other hand, the definition of low risk cannot be considered a criterion for a safe outpatient management, in fact, about one fourth of our low-risk patients required prolonged hospitalization and three of them died due to non-infectious causes. Furthermore, higher thresholds of the risk index failed to predict early discharge eligibility (data not shown). Therefore, a brief initial in-hospital management of the febrile episode followed by an outpatient continuation of antibacterial therapy in low-risk patients proved to be a safe approach. In only five instances (7%), fever recurred with the need of hospital readmission in two cases.
The choice of antimicrobial drugs employed in this study deserves some comments. The association ceftriaxone plus amikacin is not considered the gold standard as initial empiric therapy for febrile neutropenia according to international guidelines due to the low activity of ceftriaxone against some resistant gram-negative pathogens such as P. aeruginosa [26]. However, this association is widely used in the empiric treatment of febrile neutropenia, particularly in the outpatient setting, and proved to be effective against gram-negative infections [8, 16, 23–25, 29, 30]. Overall, out of seven patients who died, infection was considered the cause of death in only three cases. Considering that all infectious deaths occurred in high-risk patients with an unfavorable underlying hematologic disease, our approach to outpatient febrile neutropenia proved to be safe. We chose cefixime as the continuation oral antibiotic therapy because of its broad spectrum of activity comparable to that of ceftriaxone. This drug is not active against staphylococci and P. aeruginosa, but the low risk for infections caused by these pathogens in our population and the clinical and microbiological criteria employed in the selection of patients eligible for early discharge with cefixime treatment allowed a safe use of this antibiotic. We did exclude standard oral quinolones from this indication because most of our patients developed fever while under antibacterial prophylaxis with ciprofloxacin. To our knowledge, ours is the first experience so far reported on the use of cefixime to manage febrile neutropenia in adults. It shows that a sequential parenteral–oral treatment is a safe approach in selected low-risk patients and confirms previous experiences in the pediatric population [21, 22].
In summary, our study confirms that the MASCC risk-index is a valuable aid in the identification of high-risk febrile neutropenia patients with hematological malignancies requiring in-hospital management of the whole infectious complication. However, with regard to low-risk patients, the MASCC score was not suitable for selecting outpatient management at the onset of fever, and hospitalization in the first days of the febrile neutropenia followed by an outpatient continuation of antibacterial therapy, if indicated, appeared to be required. Cefixime could be included among the oral antibacterial drugs to be used in the outpatient treatment of adults with febrile neutropenia provided that an infection by a cefixime-resistant pathogen is excluded.
References
Rolston KV (1999) New trends in patient management: risk-based therapy for febrile patients with neutropenia. Clin Infect Dis 29:515–521
Talcott JA, Finberg R, Mayer RJ, Goldman L (1988) The medical course of cancer patients with fever and neutropenia. Clinical identification of a low-risk subgroup at presentation. Arch Intern Med 148:2561–2568
Talcott JA, Siegel RD, Finberg R, Goldman L (1992) Risk assessment in cancer patients with fever and neutropenia: a prospective, two-center validation of a prediction rule. J Clin Oncol 10:316–322
Talcott JA, Whalen A, Clark J, Rieker PP, Finberg R (1994) Home antibiotic therapy for low-risk cancer patients with fever and neutropenia: a pilot study of 30 patients based on a validated prediction rule. J Clin Oncol 12:107–114
Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, Gallagher J, Herrstedt J, Rapaport B, Rolston K, Talcott J (2000) The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 18:3038–3051
Mullen CA, Buchanan GR (1990) Early hospital discharge of children with cancer treated for fever and neutropenia: identification and management of low risk patient. J Clin Oncol 8:1998–2004
Gardembas-Pain M, Desablens B, Sensebe L, Lamy T, Ghandour C, Boasson M (1991) Home treatment of febbrile neutropenia: an empirical oral antibiotic regimen. Ann Oncol 2:485–487
Martino P, Girmenia C, Raccah R, Micozzi A, Cimino G, Sgadari C, Gentile G (1992) Single daily dose ceftriaxone plus amikacin treatment of febrile episodes in neutropenic patients attending day hospital for hematologic malignancies. Oncology 4:49–52
Rubenstein EB, Rolston K, Benjamin RS, Loewy J, Escalante C, Manzullo E, Hughes P, Moreland B, Fender A, Kennedy K, Holmes F, Elting L, Bodey GP (1993) Outpatient treatment of febrile episodes in low risk neutropenic patients with cancer. Cancer 71:3640–3646
Bash RO, Ktz JA, Cash JV, Buchanan GR (1994) Safety and cost effectiveness of early hospital discharge of lower risk children with cancer admitted for fever and neutropenia. Cancer 74:189–196
Malik IA, Khan WA, Karim M, Aziz S, Khan MA (1995) Feasibility of out-patient management of fever in cancer patients with low-risk neutropenia: results of a prospective randomised trial. Am J Med 98:224–231
Mustafa MM, Aquino VM, Pappo A, Tkaczewski I, Buchanan GR (1996) A pilot study of outpatient management of febbrile neutropenic children with cancer at low risk of bacteremia. J Pediatr 128:847–849
Sahu S, Bapna A, Pai SK, Nair CN, Kurkure PA, Advani SH (1997) Outpatient antimicrobial protocol for febrile neutropenia: nonrandomized prospective trial using ceftriaxone. Pediatr Hematol Oncol 14:205–211
Karthaus M, Egerer G, Kullmann KH, Ritter J, Jurgens H (1998) Ceftriaxone in the outpatient treatment of cancer patients with fever and neutropenia. Eur J Clin Microbiol Infect Dis 17:501–504
Hidalgo M, Hornedo J, Lumbreras C, Trigo JM, Colomer R, Perea S, Gomez C, Ruiz A, Garcia-Carbonero R, Cortes-Funes H (1999) Outpatient therapy with oral ofloxacin for patients with low risk neutropenia and fever a prospective, randomised clinical trial. Cancer 85:213–219
Minotti V, Gentile G, Bucaneve G, Iori AP, Micozzi A, Cavicchi F, Barbabietola G, Landonio G, Menichetti F, Martino P, Del Favero A (1999) Domiciliary treatment of febbrile episodes in cancer patients: a prospective randomized trial comparing oral versus parenteral empirical antibiotic treatment. Support Care Cancer 7:134–139
Mullen CA, Petropoulos D, Roberts WM, Rytting M, Zipf T, Chan KW, Culbert SJ, Danielson M, Jeha SS, Kuttesch JF, Rolston KV (1999) Outpatient treatment of fever and neutropenia for low risk pediatric cancer patients. Cancer 86:126–134
Papadimitris C, Dimopoulos MA, Kostis E, Papadimitriou C, Anagnostopoulos A, Alexopoulos G, Papamichael C, Gika D, Mitsibounas D, Stamatelopoulos S (1999) Outpatient treatment of neutropenic fever with oral antibiotics and granulocyte colony-stimulating factor. Oncology 57:127–130
Petrilli AS, Dantas LS, Campos MC, Tanaka C, Ginani VC, Seber A (2000) Oral ciprofloxscin vs. intravenous ceftriaxone administered in an outpatient setting for fever and neutropenia in low-risk pediatric oncology patients: randomised prospective trial. Med Oediatr Oncol 34:87–91
Aquino VM, Herrera L, Sandler ES, Buchanan GR (2000) Feasibility of oral ciprofloxacin for the outpatient management of febrile neutropenia in selected children with cancer. Cancer 88:1710–1714
Paganini HR, Sarkis CM, De Martino M, Zubizarreta PA, Casimir L, Fernandez C, Armada AA, Rodriguez-Brieshcke MT, Debbag R (2000) Oral administration of cefixime to lower risk febrile neutropenic children with cancer. Cancer 88:2848–2852
Shenep JL, Flynn PM, Baker DK, Hetherington SV, Hudson MM, Hughes WT, Patrick CC, Roberson PK, Sandlund JT, Santana VM, Sixbey JW, Slobod KS (2001) Oral cefixime is similar to continued intravenous antibiotics in the empirical treatment of febrile neutropenic children with cancer. Clin Infect Dis 32:36–43
Girmenia C, Moleti ML, Cartoni C, Cedrone M, De Gregoris C, De Sanctis V, Giovannini M, Latagliata R, Niscola P, Romani C, Rondinelli MB, Tosti S, Mandelli F (1997) Management of infective complications in patients with advanced hematologic malignancies in home care. Leukemia 11:1807–1812
Girmenia C, Latagliata R,Tosti S, Morano SG, Celesti F, Coppola L, Spadea A, Breccia M, Battistini R, Tafuri A, Cimino G, Mandelli F, Alimena G (1999) Outpatient management of acute promyelocytic leukemia after consolidation chemotherapy. Leukemia 13:514–517
Girmenia C, Alimena G, Latagliata R, Morano SG, Celesti F, Coppola L, Spadea A, Tosti S, Mecarocci S, D’Elia G, Tafuri A, Cimino G, Mandelli F (1999) Outpatient management of acute myeloid leukemia after consolidation chemotherapy. Role of an hematologic emergency unit. Haematologica 84:814–819
Hughes WT, Armstrong D, Bodey GP, Bow RJ, Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KVI, Shenep JL, Young LS (2002) 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis 34:730–751
Uys A, Rapoport BL, Anderson R (2004) Febrile neutropenia: a prospective study to validate the Multinational Association of Supportive Care of Cancer (MASCC) risk-index score. Support Care Cancer 12:555–560
Cherif H, Johansson E, Bjorkholm M, Kalin M (2006) The feasibility of early hospital discharge with oral antimicrobial therapy in low risk patients with febrile neutropenia following chemotherapy for hematologic malignancies. Haematologica 91:215–222
International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer (1993) Efficacy and toxicity of single daily doses amikacin and ceftriaxone versus multiple daily doses of amikacin and ceftazidime for infection in patients with cancer and granulocytopenia. Ann Intern Med 119:584–593
Rubinstein E, Lode H, Grassi C (1995) Ceftazidime monotherapy versus ceftriaxone/tobramycin for serious hospital-acquired gram-negative infections. Clin Infect Dis 20:1217–1228
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Girmenia, C., Russo, E., Carmosino, I. et al. Early hospital discharge with oral antimicrobial therapy in patients with hematologic malignancies and low-risk febrile neutropenia. Ann Hematol 86, 263–270 (2007). https://doi.org/10.1007/s00277-006-0248-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-006-0248-4