Abstract
Aplastic anemia (AA) is a rare hematopoietic stem cell disease, which can be treated with horse antilymphocyte globulin (ALG) for patients not eligible for bone marrow transplantation. ALG gives about 60% overall survival rate (OS) after 5 years, a 30% of persistent complete remission and a 20% early death rate related to failure. ALG has been incriminated in the emergence of 10 to 20% therapy-related AML/MDS (t-AML/MDS) with the usual doses. Questions remain whether higher doses of ALG could improve the response and OS rates and whether the combination with androgens is able to protect patients from t-AML/MDS. We have carried out a single institutional retrospective study of 87 AA treated with higher doses of ALG, twice the usual posology (140 mg/kg instead of 75 mg/kg), combined to androgens. The overall response rate was 77% and the OS rate at 5 years was 78%. Androgens in combination with ALG improved response and OS rates. At diagnosis, 6% of AA had an abnormal karyotype using conventional cytogenetic not related to any time-to-event. Two patients displayed a cytogenetic conversion related to the occurrence of secondary malignancies. The incidence of t-AML/MDS was 2.3% with an estimated 10-year cumulative incidence of 3.1. Our results show that higher doses of ALG combined to androgens are feasible and give results close to those recently describe with the immunosuppressive treatments including ALG associated to cyclosporine, with a low SMD/AML incidence rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aplastic anemia (AA) is a rare hematopoietic stem cell disease, which can be treated by bone marrow transplantation or immunosuppressive (IS) therapy [1, 2]. Horse antilymphocyte globulin (ALG) is the standard treatment for patients with AA who are not eligible for bone marrow transplantation [3, 4]. ALG is currently administered in Europe at the doses of 15 mg kg−1 day−1 for 5 days (75 mg/kg). The addition of cyclosporine to ALG results in improved and more rapid responses, however, without difference in overall survival (OS) [2, 5]. ALG gives an overall 60% survival rate (OS) after 5 years, a 30% of persistent complete remission (CR), and a 20% early death rate related to failure [2]. Furthermore, it has been reported a 10 to 20% late clonal complications [6, 7]. Thus, despite potential improvements to IS treatment results, increasing the doses of ALG remains suspect.
Androgens are, to date, only considered in the treatment of patients relapsing or refractory to previous IS treatments. The benefit of the combination of androgens with ALG gave conflicting results in previous studies [8, 9]. However, the association of androgens to the IS treatment was associated with a lower risk of malignancy [7].
Questions remain as to whether higher doses of ALG could improve the response and survival rates and reduce the relapse rate. Would a combination with androgens be able to protect patients from late t-AML/MDS? We report a single institutional retrospective study of AA patients treated with higher doses of ALG, in combination with androgens. The aim of this study is to show the efficiency and the safety issues of the combination of ALG at higher doses with androgens in terms of response, survival, early, and late events.
Materials and methods
Patients
We reviewed 87 newly diagnosed AA patients whose diagnosis was confirmed by aspirates and biopsies of bone marrow and were uniformly treated from January 1982 to December 2000. We excluded patients with a borderline diagnosis of AA/MDS, patients with AA post-myeloproliferative syndromes, patients presenting with paroxysmal nocturnal hemoglobinuria (PNH), and those with myelofibrosis syndrome characteristics. The median age (interquartile) was 30 (25–42), 7% were 70 years older. The M/F sex ratio was 0.93. Seventy-five percent had at least one severe cytopenia. According to Camitta and Bacigalupo criteria, 57% had a nonsevere (NSAA), 23% a severe (SAA), and 19% a very severe AA (VSAA) [10]. The degree of response was assessed when the maximum improvement in peripheral blood counts occurred; responders displayed a complete response (CR) or a partial response (PR). CR was considered when a normal hemogram was reach: hemoglobin 12 g/dl, platelets 150×109/l, and neutrophils 1.5×109/l. PR criteria were transfusion independence associated with neutrophils count greater than 0.5×109/l and platelets greater than 30×109/l. We considered death to be classifiable as “early” when it occurred 4 months after the treatment. Conventional cytogenetic evaluation was systematically carried out at diagnosis, and repeated every time patients presented with cytopenias throughout their disease history. We considered a clone to be abnormal when the karyotype displayed at least two metaphase cells with the same abnormality.
Treatments
The patients were treated when their vital prognosis was engaged even when they displayed only one severe cytopenia. Patients received in first line: 6 to 8 h IV infusion with central catheter of horse ALG preparation (Lymphoglobuline, Institut Merieux, Lyon, France), used at 20 mg kg−1 day−1 for 7 days (140 mg/kg). Methylprednisolone at 1 mg kg−1 day−1 covering the overall ALG treatment was systematically associated. Sixty-four (74%) patients were treated nonrandomly in combination with oral norethandrolone (Nilevar: 1 mg kg−1 day−1), for 6 months before progressive reduction in dosage. The remaining 23 patients did not receive androgens according to their physician’s decision or because they refused. Cyclosporine was added in case of failure or relapse after a second course of ALG. No growth factor or other IS treatment were associated in the first line treatment. Patients received transfusions with irradiated red blood cell and platelets.
Statistical analysis
The continuous variables were characterized through median and interquartiles. Correlations were tested through the Spearman correlation coefficient. The parameters in Table 1 were examined for their prognostic value on overall survival. Event-free survival (EFS) was calculated for the overall population from the start of treatment until events occurred (refractory disease, relapse, and death). Survivals were evaluated through Kaplan–Meier estimates [11] and compared through Log-rank tests [12]. The estimate of the relative risk, and its 95% confidence interval (CI 95%), were estimated through the proportional hazard model [13]. All analyses were performed with SPSS software [14].
Results
In the overall population
After a median follow-up of 112 months (CI 95%, 90–134), 22 (25%) patients died, 9 of them died prematurely. The overall response and CR rates to the first ALG were 77% (n=67) and 64.4% (n=56), respectively. Twenty (23%) patients failed to obtain a response to the first line of treatment. The relapse rate was 39% (n=26). AA severity was not correlated to any response rates and survival times. The failure to respond was closely associated to the incidence of death and particularly early death. Fifteen (68%) refractory patients died because of infections and hemorrhage compared to five (22%) in the responding group (P<0.0001). The two remaining patients died years after their diagnostic from other noncancer diseases while in CR. The absence of response was correlated with the reticulocyte level, the platelet, and the white blood cell count at diagnosis (r=0.302, P=0.007; r=0.234, P=0.029; r=0.220, P=0.041), respectively. Nine (10%) patients had serum sickness, requiring treatment with corticosteroids. Six (7%) had hip aseptic osteonecrosis. Five (6%) patients displayed an increase in cytolytic hepatic laboratory values and required cessation of the androgen treatment, which could be reintroduced but with reduced doses. All patients had sonography survey of the liver and none of them presented with adenoma or peliosis of the liver. All genders presented with gain of weight, muscle cramps but women presented more specifically with skin changed such as increase pilosity, amenorrhea, and low voice change. All side effects resolved but not the change in voice.
In the subgroup treated with androgens
The characteristics and results from patients treated in combination with androgens compared to those treated with ALG alone are summarized in Table 1. Fifty-three (83%) patients treated with androgens obtained a response, compared to 14 (61%) patients without androgens (P=0.032). The CR rates in these two groups were 46 (72%) and 10 (43.5%) patients, respectively (P=0.015). Twelve (19%) patients died in the group treated with androgens and 10 (43.5%) patients in the group without (P=0.019). There is a trend for the female to benefit more from androgens in term of response but not survival (data not shown).
Prognostic factors
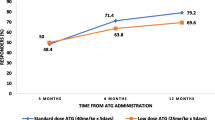
The median OS was not reached, the mean being 231 months +/−15. The median EFS was 9 months +/−2.7. The parameters significantly affecting OS as shown by univariate analysis are presented in Table 2. The relapse rate was not significant (P=0.21). The combination of androgens and an IS regimen was predictive for OS, but not for EFS and PFS (P=0.0056, P=0.104, and P=0.164, respectively). The probability of overall survival at 5 and 15 years are 84 and 64%, 77.5 and 47%, then were stable thereafter in the two groups with and without androgens, respectively. The relative risk (CI 95%) of death was 3.09 (1.33–7.19), with a mean survival time of 128±23 months for patients without androgens vs 252±15 months for those with androgens (P=0.0056) (Fig. 1).
Cytogenetic and t-AML/MDS
Three patients (6%) had the following abnormal karyotypes at diagnosis: 46,XX,del(20)(q12), 46,XX,del(5)(q22q32), and 47,XX, +mar(der6? der8?). Two patients had a cytogenetic conversion with a monosomy 7 while having been normal at diagnosis. The karyotype at diagnosis had no prognostic impact on survivals and secondary leukemia incidence. No patient with a relapse or a refractory disease, i.e., retreated with IS treatment, developed cytogenetic abnormalities. Two patients (2.3%) presented secondary malignancies (1 AML and 1 MDS) with a monosomy 7 conversion while having been normal at diagnosis. Both were in first CR and had received androgens. The estimated 20-year cumulative incidence of t-AML/MDS rates was 6.5.
Discussion
These results have to be interpreted with caution because of the nonprospective results and the small groups. In our study, the higher doses of ALG in combination with androgens gave a higher than expected response and estimated survival rates. It seems that this combination in first line of therapy gave comparable results to the combination of ALG and cyclosporine [1, 5]. The regimen was well tolerated when accompanied by adequate management of septic syndromes and transfusion protocols.
We found that androgens introduced in combination with higher doses of ALG in first line treatment significantly increased both the overall response and CR rates and the OS [8, 15]. However, both ALG and androgens failed to improve the refractory population results [8]. Some have previously reported a significantly greater response rate with the addition of androgens to ALG, without improvement in survival [8, 15]. Others have reported no benefit of androgens either in response or in survival [9, 16], and an increased incidence of relapses compared to ALG alone, reflecting an androgen dependency [17]. For several patients, we also observed a transient reduction in blood count during androgen decreased, corresponding to dependency. The cytopenias corrected when doses were raised back to the previous threshold. The main consequence was that many patients received androgens for longer than 6 months. However, no man or woman treated with androgens has displayed androgen-induced side effects greater than grade 1 and 2.
In our study, the incidence rate of t-AML/MDS is lower than those previously published. Nevertheless, this rate is higher than the expected incidence rate in a normal population, showing that IS therapy likely increases the risk. The main differences from other studies come from the association of androgens to our regimen, the lower median age, and less severe cases of AA. In our study, cytogenetic and bone marrow histology at diagnostic combined with the long follow-up was in favor of AA in any case [18, 19]. At the opposite, the acquisition of monosomy 7 provided strong evidence of a clonal evolution [18, 20]. The incidence rate of cytogenetic conversion in our study is lower than previously published, perhaps because rechecking for cytogenetic anomalies was not a routine procedure.
Higher doses of ALG in combination with androgens are a feasible treatment and have provided interesting results without increasing the late clonal complication incidence rate. In consequence, androgens could be an alternative to cyclosporine in case of cyclosporine contraindication.
References
Killick SB, Marsh JC (2000) Aplastic anaemia: management. Blood Rev 14:157–171
Frickhofen N, Rosenfeld SJ (2000) Immunosuppressive treatment of aplastic anemia with antithymocyte globulin and cyclosporine. Semin Hematol 37:56–68
Bacigalupo A, Broccia G, Corda G, Arcese W, Carotenuto M, Gallamini A, Locatelli F, Mori PG, Saracco P, Todeschini G et al (1995) Antilymphocyte globulin, cyclosporin, and granulocyte colony-stimulating factor in patients with acquired severe aplastic anemia (SAA): a pilot study of the EBMT SAA Working Party. Blood 85:1348–1353
Marsh JC, Gordon-Smith EC (1998) Treatment options in severe aplastic anaemia. Lancet 351:1830–1831
Ball SE (2000) The modern management of severe aplastic anaemia. Br J Haematol 110:41–53
Young NS, Maciejewski J (1997) The pathophysiology of acquired aplastic anemia. N Engl J Med 336:1365–1372
Socie G, Gluckman E (2000) Cure from severe aplastic anemia in vivo and late effects. Acta Haematol 103:49–54
Bacigalupo A, Chaple M, Hows J, Van Lint MT, McCann S, Milligan D, Chessells J, Goldstone AH, Ottolander J, van’t Veer ET et al (1993) Treatment of aplastic anaemia (AA) with antilymphocyte globulin (ALG) and methylprednisolone (MPred) with or without androgens: a randomized trial from the EBMT SAA working party. Br J Haematol 83:145–151
Paquette RL, Tebyani N, Frane M, Ireland P, Ho WG, Champlin RE, Nimer SD (1995) Long-term outcome of aplastic anemia in adults treated with antithymocyte globulin: comparison with bone marrow transplantation. Blood 85:283–290
Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT, Congiu M, De Planque MM, Ernst P, McCann S et al (1988) Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol 70:177–182
Kaplan E, Meier P (1958) Non parametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170
Cox DR (1972) Regression models and life-tables. J R Stat Soc B 34:187
SPSS (1986) SPSS/PC+: statistical package for social science, user’s guide. SPSS, Chicago, IL
Kaltwasser JP, Dix U, Schalk KP, Vogt H (1988) Effect of androgens on the response to antithymocyte globulin in patients with aplastic anaemia. Eur J Haematol 40:111–118
Champlin RE, Ho WG, Feig SA, Winston DJ, Lenarsky C, Gale RP (1985) Do androgens enhance the response to antithymocyte globulin in patients with aplastic anemia? A prospective randomized trial. Blood 66:184–188
Schrezenmeier H, Marin P, Raghavachar A, McCann S, Hows J, Gluckman E, Nissen C, van’t Veer-Korthof ET, Ljungman P, Hinterberger W et al (1993) Relapse of aplastic anaemia after immunosuppressive treatment: a report from the European Bone Marrow Transplantation Group SAA Working Party. Br J Haematol 85:371–377
Maciejewski JP, Risitano A, Sloand EM, Nunez O, Young NS (2002) Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood 99:3129–3135
Socie G, Rosenfeld S, Frickhofen N, Gluckman E, Tichelli A (2000) Late clonal diseases of treated aplastic anemia. Semin Hematol 37:91–101
Mikhailova N, Sessarego M, Fugazza G, Caimo A, De Filippi S, van Lint MT, Bregante S, Valeriani A, Mordini N, Lamparelli T, Gualandi F, Occhini D, Bacigalupo A (1996) Cytogenetic abnormalities in patients with severe aplastic anemia. Haematologica 81:418–422
Camitta B, O’Reilly RJ, Sensenbrenner L, Rappeport J, Champlin R, Doney K, August C, Hoffmann RG, Kirkpatrick D, Stuart R, Santos G, Parkman R, Gale RP, Storb R, Nathan D (1983) Antithoracic duct lymphocyte globulin therapy of severe aplastic anemia. Blood 62:883–888
Acknowledgements
We wish to thank Pr G. Socie for his help in the preparation of the manuscript. We wish to thank Allen Ho who kindly reviewed the manuscript. Xavier Leleu and Louis Terriou contributed equally to this work.
Conflict of interest statement
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leleu, X., Terriou, L., Duhamel, A. et al. Long-term outcome in acquired aplastic anemia treated with an intensified dose schedule of horse antilymphocyte globulin in combination with androgens. Ann Hematol 85, 711–716 (2006). https://doi.org/10.1007/s00277-006-0152-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-006-0152-y