Abstract
We investigated erythropoietin (Epo) response in a cohort of diabetic patients with various types of anemia to approach the pathogenesis of some cases of “unexplained” anemia encountered among diabetics. Serum Epo levels were determined totally in 747 evaluable subjects with normal renal and hepatic function, of whom 694 had anemia. Among anemic patients, 237 were diabetics, while among the 53 nonanemic persons, there were also 21 diabetics. Diabetic and nondiabetic subjects were uniformly balanced in relation to their demographic features and were categorized according to the etiology of their anemia. Hemoglobin (Hb) did not differ between diabetic and nondiabetic subjects in all the etiological groups and in the whole population. Diabetic patients had significantly lower serum Epo levels as compared to nondiabetics (36.5±61 vs 69.4±191 IU/ml, p<0.0001), and this was true for all etiologic groups of anemia with the exception of patients with myeloproliferative disorders and those with megaloblastic anemia. The natural logarithmic (ln)–Epo×Hb component was used as an index of response to anemia and was found to be significantly decreased in almost all subgroups of diabetic patients. Serum Epo levels were also negatively correlated with the percentage of glycosylated Hb, HbA1C (r=−0.446), and the correlation was stronger with the ln of serum Epo (r=−0.638, p<0.001). Inappropriately low serum Epo level is a uniform feature in patients with type II diabetes mellitus and may represent a constitutive blunted response to anemia or an altered metabolic rate of Epo, probably as a result of abnormal glycosylation of the cytokine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Noninsulin-dependent diabetes mellitus (NIDDM) is a systemic disease which involves many organs of the body. Anemia is mostly associated with various complications of diabetes, and its etiology is multifactor. Nutritional or secondary to occult blood loss iron deficiency, megaloblastic anemia associated with the presence of antiparietal antibodies, B12 malabsorption or folate deficiency, and anemia of renal failure are main causes, although other less common factors such as hemolysis due to microangiopathy or to drug-induced immune mechanisms, marrow hypoplasia, and increased membrane rigidity [1] have been implicated. There are, however, some cases where no etiology of anemia can be found.
In a recently published study, the incidence of anemia among 1,054 diabetic patients was 12.5%, but in the majority of cases, it was unrecognized, undetected, or underestimated [2]. In a previous study, searching risk factors for unexpected hospitalizations in diabetic patients, anemia was such a predictive factor [3].
In the majority of the anemic patients, there is an inverse relationship between hemoglobin (Hb) concentration and serum erythropoietin (Epo) levels [4]. This is not, however, the case in patients with renal failure [5] or in anemia of chronic diseases [6, 7], where serum Epo is inappropriately low for the degree of anemia. In anemic patients with type I diabetes, but without overt renal failure, serum Epo has been found to be inappropriately low, as compared to that of nondiabetic patients with glomerulonephritis [8], and this finding has been postulated to represent a sign of coexisted autonomic neuropathy [9, 10], while for patients with diabetic nephropathy, serum Epo reflects the severity of the disease [11].
The observation that many patients with as yet uncomplicated diabetes and anemia had inappropriately low serum Epo levels for the degree of their anemia [12], as well as the fact that no comparative study to nondiabetic anemic patients existed in literature, prompted us to perform a more systemic study in the time period between 1995 and 2004 and to compare diabetic and nondiabetic patients with anemia of different etiologies and severities in relation to their serum Epo levels. We also correlated the percentage of glycosylated Hb in diabetic patients, a marker of appropriate control of the disease, with serum Epo levels. While our investigation was in progress, several studies were published reporting reduced Epo response to anemia in relatively small numbers of patients with type I [13, 14] and type II [15, 16] diabetes.
Patients and methods
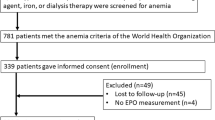
Plasma Epo was measured totally in 908 subjects, including 851 anemic patients (308 diabetics and 543 nondiabetics), who were evaluated in the hematology out-patient clinic for anemia of various severities and etiologies. In these anemic patients, the determination of plasma Epo levels was part of their baseline workout. Plasma Epo was also measured in an additional 57 subjects, including 24 nonanemic diabetic patients of the diabetes out-patient clinic of the Department of Internal Medicine, as well as in 33 age-matched normal volunteers after informed consent.
All patients and controls included in the study had normal renal function as demonstrated by a normal serum creatinine level (≤1.6 mg/dl). Sixty two patients (28 diabetics and 34 nondiabetics) with serum creatinine more than 1.6 mg/dl were excluded from the analysis. In an additional 47 anemic patients (22 diabetics and 25 nondiabetics) with a borderline serum creatinine (1.5–1.6 mg/dl), calculation of creatinine clearance was performed, and 28 of them (15 diabetics and 13 nondiabetics) with values less than 50 ml/min were also excluded from the study. Accordingly, 13 patients (11 diabetic and 2 nondiabetic) with subclinical nephropathy estimated by the presence of macroalbuminuria, even if they had normal serum creatinine, as well as 34 patients in total (12 diabetics and 22 nondiabetics) receiving angiotensin-converting enzyme (ACE) inhibitors for the control of their blood pressure and/or cardiac function, were also not included in the study. Finally, 19 patients (7 diabetics and 12 nondiabetics) were excluded due to inadequate data from their medical history. Thus, in total, 747 subjects, including 694 anemic patients (among them 237 diabetics and 457 nondiabetics) and 53 nonanemic subjects (21 diabetics and 32 control persons), were analyzed, while the remaining 157 anemic patients and 4 nonanemic persons (three diabetics and one normal control) were excluded from the analysis.
The majority of diabetic patients (251/258 or 97.3%) had adult-onset noninsulin-dependent DM treated either with an oral administration of antidiabetics (79/258 or 30.6%), insulin (163/258 or 63.2%), or with both modalities simultaneously (13/258 or 5%). Three patients (1.2%) did not receive any kind of treatment.
For every person tested, a detailed medical history was obtained, followed by a physical examination, and a complete laboratory investigation, including complete blood counts with evaluation of a blood smear, measurement of reticulocytes, erythrocyte sedimentation rate, as well as urinalysis and a basic biochemical profile, including blood urea nitrogen (BUN), serum creatinine, electrolytes, hepatic enzymes, total and fractionated bilirubin, lactate dehydrogenase (LDH), uric acid, total protein and albumin, iron, total iron binding capacity (TIBC), ferritin, B12, folate, and Epo level measurement. Blood samples were obtained between 8 and 9:30 a.m., and Epo determinations were performed using radioimmunoassay. The linear segment of the curve was between the values of 0 and 315 mIU/ml. When a sample was measured above the linear segment of the curve, the measurement was repeated from the same blood sample after a 1:10 dilution of the plasma.
In many patients with an initially obscure etiology of anemia, additional diagnostic procedures, including Hb electrophoresis, serum protein electrophoresis, erythrocyte enzyme determination, acidified serum lysis test, serum thyroid hormone profile, and a bone marrow aspirate with a subsequent trephine biopsy were performed. The last two procedures were also performed for staging reasons in all patients with lymphoproliferative and myeloproliferative disorders.
For the estimation of the glucose control status in the diabetic-patient population, determination of glycosylated Hb by liquid chromatography was performed in 141 (55%) of the 258 diabetic patients at the same time point with the rest-patient work-up. Patients with HbA1c determinations at different time points than that of the evaluation of anemia and serum Epo level determination were not included in the analysis of relationship between serum Epo levels and glucose control status.
Statistical package SPSS V.11 was used for the statistical analysis. Mann–Whitney U test was applied to compare the Hb and Epo levels between the various groups resulting from the etiology of anemia. Pearson’s χ 2 was applied for comparisons of frequencies and percentages. The effect of Hb on Epo levels was assessed by regression analysis after natural logarithmic (ln) transformation of independent variable to moderate the skewed distribution. The 5% level was chosen for the assessment of the statistical significance in all tests.
Results
Clarification of the etiology of the anemia
After the clarification of the etiology of the anemia, patients were classified as presented on Tables 1 and 2. There was no difference in age and sex distribution between the two groups of subjects tested. The diabetic patient population consisted of 147 males and 111 females (male to female ratio 1.324) with a median age of 64 years (age range 19–91 years). The nondiabetic patient population consisted of 278 males and 211 females [male to female ratio 1.317, χ 2=0.16, p is not significant (NS)] with a median age of 65 years (range 18–89 years). In almost all etiologic subgroups, male and female patients were uniformly distributed. For each diabetic patient, roughly two nondiabetics with anemia of the same etiology were compared.
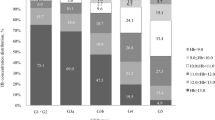
In the anemic patient population, Hb values ranged between 4.1 and 12 g/dl among the diabetics and between 3.7 and 12 g/dl among the nondiabetics. There was a uniform distribution of the severity of anemia in both groups (Pearson χ 2 6.06, p=0.810). Forty one (15.9%) out of 258 diabetic patients and 73 (14.9%) out of 489 nondiabetic patients had severe anemia (Hb<8 g/dl); the corresponding numbers for moderate anemia (Hb 8–9.9 g/dl) were 41.1% (106/258) and 45.2% (221/489), respectively, and for mild anemia (Hb 10–12 g/dl), the corresponding numbers were 30.2% (78/258) for diabetics and 28.2% (138/489) for the nondiabetic population. There were also 12 (4.7%) marginally anemic patients (Hb 12–12.5 g/dl) among the diabetic group and 25 (5.1%) similarly anemics among the nondiabetic group. Finally, the nonanemic population (Hb >12.5 g/dl) consisted 8.1% (21/258 patients) of the diabetic group and 6.5% (32/489) of the nondiabetic group.
Serum Epo levels in anemic patients with or without NIDDM
Table 3 shows the Hb levels and serum Epo concentrations of the 694 diabetic and nondiabetic patients suffering from anemia of various causes, after the clarification of the etiology of their anemia, and of the 53 nonanemic subjects. The Hb concentration of every etiologic group was not different when compared between the diabetic and nondiabetic patient populations. In contrast to Hb, serum Epo levels were significantly lower among the diabetic population than among the nondiabetics in almost all the anemic groups tested, especially when a large number of patients had been included, and in the whole patient population. Only in two etiologic groups, namely, in the group of patients with myeloproliferative disorders and in that of patients with megaloblastic anemia, did the difference not reach the level of statistical significance or was of borderline significance, probably because of the relative small number of patients tested, and due to a large variation of values.
When all anemic patients, irrespective of the etiology of their anemia, were compared, serum Epo levels were very significantly lower in the diabetic, than in the nondiabetic, patient population (Mann–Whitney U test z=−7.38, p<0.001), although the Hb values of the two groups were comparable (z=−0.46, p=0.645).
Serum Epo levels in nonanemic persons
When we compared serum Epo levels in the 21 diabetic patients without anemia and in the 32 healthy persons, matched for age and sex and used as controls (Table 3), we detected a significantly decreased serum Epo level among the diabetic group as compared to the control population (Mann–Whitney U test p=0.035). Again, the Hb levels of the two groups were similar (p=0.780).
Association between Hb and Epo
In both diabetic (group I) and nondiabetic (group II) subjects, there was a significant negative correlation between serum Epo levels and Hb concentration after the values of the two variables were ln-transformed (Spearman’s r, group I=−0.611, p<0.001; group II=−0.684, p<0.001; Fig. 1). To further evaluate the effect of Hb on Epo levels, we tested the regression of ln-Hb on ln-Epo in both groups of subjects studied. The resulting regression lines are described by the following equations:
and
Hb values are expressed in g/dl, and Epo values are expressed in mIU/ml.
To assess the effect of age, we inserted that factor in each regression, but the resulting F change was insignificant (p>0.05), which implies that there is no effect of age in either group. The comparison of the two regression lines (performed by fitting a single model containing a dummy variable, z, taking the values 0 for diabetics and 1 for nondiabetics) showed that they are parallel but with different intercepts that are lower in the diabetic group of patients (p<0.05). This finding suggests that serum Epo levels in the diabetic population tested were consistently lower than the corresponding levels of the nondiabetic population at all levels of hemoglobin, i.e., at all degrees of severity of the anemia. The above findings are presented in Fig. 1.
Serum Epo levels and percentage of glycosylated Hb
To investigate whether the impaired serum Epo levels in relation to blood Hb concentration which we observed in anemic diabetic patients, irrespective of the severity and the etiology of their anemia, was influenced by the level of glucose control achieved by the patient, we correlated serum Epo levels with the percentage of glycosylated Hb (fragment HbA1C), which was measured in 141 of the diabetic patients at the same time point. The two variables were regressed with a negative correlation (r=−0.446, p<0.01). The correlation was stronger between the ln of serum Epo and HbA1C (r=−0.638, p<0.001). The fitted model was as follows: ln-Epo=6.23-0.52×HbA1C (R 2=0.41, p<0.001), (Fig. 2).
Discussion
In this study, we detected significantly decreased serum Epo levels for the degree of Hb in diabetic patients with anemia of almost any etiology, except of megaloblastic anemia, and anemia of myeloproliferative disorders. This is in accordance with a recent report of 35 diabetic patients with anemia of uncertain etiology without advanced nephropathy, published during the accrual of our patients, in which reduced Epo responsiveness to anemia was found [15]. The authors hypothesized that decreased Epo levels may reflect early renal interstitial damage affecting the Epo-synthesizing cells. Cotroneo et al. have found a blunted Epo response in 10 of 13 anemic patients studied with type I diabetes [14]. An inhibitory activity of proinflammatory cytokines and of advanced glycation end products [10], as well as loss of Epo in the urine, have been proposed as contributing factors. Anemia with blunted Epo response was present in 38% of 84 patients with primary autonomic failure [9], and autonomic neuropathy has been suggested as a possible cause of anemia in patients with type I diabetes without overt renal failure [10]. In another study, diabetes itself was an independent risk factor for the development and/or deterioration of anemia among nondialyzed patients with renal failure [17].
Our results indicate that inappropriately low serum Epo is a rather uniform feature of NIDDM accompanying not only anemic but also nonanemic diabetics, compared to nondiabetic persons, matched for Hb, age, and sex. The observed inverse correlation between HbA1c and serum Epo probably suggests a relationship between poor glycemic control and blunted Epo response. If this is true, then many otherwise “unexplained” cases of anemia accompanying diabetes can be explained, and a better glucose control can ameliorate Epo response, thus improving anemia in a proportion of diabetics. A prospective study is certainly needed to address this issue. However, many additional factors such as subclinical nephropathy, affecting the paraglomerular apparatus, local microangiopathy, and/or disturbances of the autonomic nervous system of the kidney, all rather common lesions in long-standing and poorly controlled NIDDM, can affect Epo production.
Besides chronic renal failure [5], a blunted Epo response is observed in the anemia of chronic diseases commonly encountered in inflammatory, infectious, autoimmune, and neoplastic diseases. The pathogenesis of this anemia is not fully understood, although negative regulators of erythropoiesis such as tumor necrosis factor (TNF)α, transforming growth factor (TGF)β, and interferon (IFN)γ are considered to play an important role [6, 7]. In many of our patients with coexistent chronic/systemic, benign, or malignant diseases, the presence of NIDDM was an additional adverse prognostic factor for the development and perpetuation of anemia. Similar findings have also been reported by others [17]. We therefore suggest that NIDDM should be included among the causes producing a blunted Epo response and leading to a normochromic, sideroachrestic anemia.
Another possible explanation might be a more rapid clearance of Epo from the circulation. Epo is a glycoprotein, and about 40% of its molecule consisted of N- and O-linked sugar chains. There are three N-linked sugar chains at asparagine (Asn)24, Asn38, and Asn83 and one O-linked chain in each Epo molecule [18, 19]. The addition of various sugars at these positions, and especially the terminal sialylation, confers stabilization to the molecule, while the nonglycosylated protein, although retaining its biologic activity, has a short half-life and is rapidly degraded by the hepatocytes [19, 20]. In addition to enzymatic (covalent) glycosylation, Epo molecule is noncovalently glycosylated, mainly at Asn and lysine (Lys) residues, and the rate of this process is highly dependent upon serum glucose levels. In diabetic patients, nonenzymatic glycosylation is a global, steady-state process, which affects both structural and functional proteins [21] and alters the physicochemical properties of the exposed proteins, and in particular, modulates its catabolic rate [22]. Although variability in the degree of glycosylation may exist, this can be average-estimated by the determination of Hb glycosylation, i.e., by HbA1C. It is therefore possible that in diabetic patients, Epo may be produced normally, but catabolized faster, as a result of increased or abnormal glycosylation. Supporting the above are the findings of Bosman et al., who demonstrated a normal Epo response to hypoxia in five diabetic patients with inappropriately low serum Epo levels indistinguishable to that of nine normal subjects [23]. Clearly, a biochemical study of circulating Epo molecule in diabetic patients appears mandatory to address this hypothesis.
A blunted Epo response in NIDDM, albeit potentially important for the pathogenesis of anemia, should not lead to underestimation of additional factors, which might contribute to anemia in these patients, including iron and B12 deficiency, hemolysis, and coexistent infectious complications. Therefore, treatment of anemia in diabetic patients must always take into account all other possible etiologic factors and treat them accordingly [24]. There are sporadic reports of therapeutic use of rh-Epo in diabetics. Interestingly, rh-Epo appears to affect glucose control through many different mechanisms, and there are conflicting data in literature [25]. In our population, only 2 of the 14 diabetic patients with persistent substantial anemia of chronic diseases were administered rh-Epo 100 IU/kg three times weekly. They both responded well, increasing their Hb >2 g/dl after 2 months of treatment. Thus, in selected cases of anemia in diabetics, when no other etiology is found, even when prominent renal failure is absent, treatment with rh-Epo can be effective and should be considered as a therapeutic option.
References
Symeonidis A, Psiroyannis A, Kyriazopoulou V, Kapatais-Zoumbos K, Missirlis Y, Zoumbos N (2001) Impairment of erythrocyte viscoelasticity is correlated with levels of glycosylated haemoglobin in diabetic patients. Clin Lab Haematol 23:103–109
Stevens PE, O’Donogue DJ, Lameire NR (2003) Anemia in patients with diabetes: unrecognized, undetected and untreated? Curr Med Res Opin 19:395–401
Smith DM, Norton JA, Roberts SD, Maxey WA, McDonald CJ (1983) Unexpected hospital admissions among patients with diabetes mellitus. Arch Intern Med 143:41–47
de Klerk G, Rosengarten PC, Vet RJ, Goudsmit R (1981) Serum erythropoietin (EST) titers in anemia. Blood 58:1164–1170
Urabe A, Saito T, Fukamachi H, Kubota M, Takaku F (1987) Serum erythropoietin titers in the anemia of chronic renal failure and other hematological states. Int J Cell Cloning 5:202–208
Baer AN, Dessypris EN, Goldwasser E, Krantz SB (1987) Blunted erythropoietin response to anaemia in rheumatoid arthritis. Br J Haematol 66:559–564
Miller CB, Jones RJ, Piantadosi S, Abeloff MD, Spivak JL (1990) Decreased erythropoietin response in patients with the anemia of cancer. N Engl J Med 322:1689–1692
Bosman DR, Winkler AS, Marsden JT, McDougall IC, Watkins PJ (2001) Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care 24:495–499
Hadjadj S, Torremocha F, Fanelli A, Brizard A, Baues M, Marechaud R (2001) Erythropoietin-dependent anemia: a possible complication of diabetic neuropathy. Diabetes Metab 27:383–385
Winkler AS, Marsden J, Chaudhuri KR, Hambley H, Watkins PJ (1999) Erythropoietin depletion and anaemia in diabetes mellitus. Diabet Med 16:813–819
Inomata S, Itoh M, Imai H, Sato T (1997) Serum levels of erythropoietin as a novel marker reflecting the severity of diabetic nephropathy. Nephron 75:426–430
Kojima K, Totsuka Y (1995) Anemia due to reduced serum erythropoietin concentration in non-uremic diabetic patients. Diabetes Res Clin Pract 27:229–233
Ricerca BM, Todaro I, Caputo S et al (1999) Blunted erythropoietin response to anemia in patients with type 1 diabetic patients. Diabetes Care 22:647
Cotroneo P, Maria Ricerca B, Todaro L et al (2000) Blunted erythropoietin response to anemia in patients with type 1 diabetes. Diabetes Metab Res Rev 16:172–176
Yun YS, Lee HC, Yoo NC et al (1999) Reduced erythropoietin responsiveness to anemia in diabetic patients before advanced diabetic nephropathy. Diabetes Res Clin Pract 46:223–229
Craig KJ, Williams JD, Riley SG et al (2005) Anemia and diabetes in the absence of nephropathy. Diabetes Care 28:1118–1123
Ihimura E, Nishizawa Y, Okuno S et al (1998) Diabetes mellitus increases the severity of anemia in non-dialyzed patients with renal failure. J Nephrol 11:88–91
Rush RS, Derby PL, Smith DM et al (1995) Micro-heterogeneity of erythropoietin carbohydrate structure. Anal Chem 67:1442–1452
Misaizu T, Matsuki S, Strickland WT, Takeuchi M, Kobata A, Takasaki S (1995) Role of antennary structure of N-linked sugar chains in renal handling of recombinant human erythropoietin. Blood 86:4097–4104
Rahbek-Nielsen H, Roepstorff P, Reischl H, Wozny M, Koll H, Haselbeck A (1997) Glycopeptide profiling of human urinary erythropoietin by matrix-assisted laser desorption/ionization mass spectrometry. J Mass Spectrom 32:948–958
Guthrow EC, Morris MA, Day JF, Thorpe SR, Baynes JW (1979) Enhanced non-ezymatic glycosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci U S A 76:4258–4261
Watala C (1992) Hyperglycemia alters the physicochemical properties of proteins in erythrocyte membranes of diabetic patients. Int J Biochem 24:1755–1761
Bosman DR, Osborne CA, Marsdent JT, Macdougall IC, Gardner WN, Watkins PJ (2002) Erythropoietin response to hypoxia in patients with diabetic autonomic neuropathy and non-diabetic chronic renal failure. Diabet Med 19:65–69
Dikow R, Schwenger V, Schömig M, Ritz E (2002) How should we manage anemia in patients with diabetes? Nephrol Dial Transplant 17(Suppl 1):67–72
Rigalleau V, Blanchetier V, Aparichio M et al (1998) Erythropoietin can deteriorate glucose control in uremic non-insulin dependent diabetic patients. Diabetes Metab 24:62–65
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Symeonidis, A., Kouraklis-Symeonidis, A., Psiroyiannis, A. et al. Inappropriately low erythropoietin response for the degree of anemia in patients with noninsulin-dependent diabetes mellitus. Ann Hematol 85, 79–85 (2006). https://doi.org/10.1007/s00277-005-1102-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-005-1102-9