Abstract
Recent studies have demonstrated that direct cell-to-cell interaction is one of the microenvironment factors for transdifferentiation of adult stem cells into cardiomyocytes. We investigated whether transdifferentiation of mesenchymal stem cells (MSCs) into cardiomyocytes was dependent on developmental stages of cocultured cardiomyocytes, and direct cell-to-cell interaction was essential for transdifferentiation. MSCs were isolated from adult rat and cocultured in four different ways: (1) with neonatal cardiomyocytes, (2) with adult cardiomyocytes, (3) with neonatal cardiomyocytes on the cell culture inserts, and (4) with the conditioned medium from neonatal cardiomyocytes. After 5 days of coculture with neonatal cardiomyocytes, 9.40±1.15% of 1,1′-dioctadecyl-1-3,3,3′,3′-tetramethylindocarbocyanine perchlorate labeled MSCs expressed sarcomeric-α-actinin. Immunocytochemistry showed that only these MSCs expressed the cardiac markers and were not observed with other coculture condition as well as conditioned medium. Calcein-AM labeling of cardiomyocytes showed gap junctional communication between 56.1±2.0% of MSCs (24 h after labeling, n=5) and neonatal cardiomyocytes. These findings suggest that MSCs are capable of differentiating into cardiomyocytes when directly cocultured with neonatal cardiomyocytes by cell-to-cell interaction, but not with adult cardiomyocytes or conditioned medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction leads to cardiomyocyte loss and, consequently, deterioration of cardiac function. Because cardiomyocytes have a limited ability for self-renewal, and thus replace damaged tissue, cell transplantation is a promising therapy to regenerate damaged cardiac tissue. These cells have the capacity to differentiate to the desired cell type under appropriate stimuli, have a limited capacity to multiply, and should be capable of functional integration into the host myocardium. Bone-marrow-derived mesenchymal stem cells (MSCs) are differentiated into cardiomyocytes by chemicals [1], growth factors [2], and also can be differentiated into cardiomyocytes by microenvironment in vivo [3]. Wang et al. [3] demonstrated that the MSCs injected into periinfarcted myocardium differentiated into cardiomyocytes within the scar as the result of cellular migration, suggesting the myocardium microenvironment supports growth and induce cardiomyogenic differentiation of MSCs after implantation. However, the mechanisms and the signals to induce MSCs to undergo cardiomyogenic differentiation within the myocardial environment are not fully understood in the described experiments because of in vivo nature. To improve the heart function after myocardial infarction by cell transplantation, these mechanisms and the signals require to be solved. There are some candidate signals that direct MSCs to differentiate into cardiomyocytes that are physical factors as well as chemicals or unknown soluble factors. Some studies demonstrated human endothelial progenitor cells (EPCs) [4], skeletal muscle-derived cells [5], and bone marrow (BM) cells [6] can transdifferentiate into cardiomyocytes when cocultivated with neonatal cardiomyocytes, suggesting that direct cell-to-cell contact is one of the environmental factors. In addition, Ijima et al. [5] reported that contraction of neighboring cardiomyocytes, as well as direct cell-to-cell contact, is necessary for transdifferentiation of skeletal muscle cells into cardiomyocytes. The contraction was also one of the differences between neonatal and adult cardiomyocyte cultured in vitro, which was only seen in neonatal cardiomyocyte [7].
We investigated whether transdifferentiation of MSCs into cardiomyocytes was dependent on developmental stages, and direct cell-to-cell interaction was essential for transdifferentiation of MSCs into cardiomyocytes. To elucidate these questions, we used a coculture system that MSCs were cultured in four different ways: (1) with neonatal cardiomyocytes, (2) with adult cardiomyocytes, (3) with neonatal cardiomyocytes on the cell culture inserts (double chamber system), or (4) with conditioned medium from neonatal cardiomyocytes. The results of the present study demonstrate that MSCs are capable of differentiating into cardiomyocytes when directly cocultured with neonatal cardiomyocytes, but not with adult cardiomyocytes or with conditiond medium from neonatal cardiomyocytes.
Materials and methods
Bone marrow stem cell preparation
Bone marrow cells were harvested from the femur and tibia of adult F344 rats weighing 200–210 g. Isolation and primary culture of MSCs were performed according to Caplan’s method [5]. Briefly, the BM plugs were hydrostatically expelled from the bones with Isocove’s modified Dulbecco’s medium (IMDM) containing 10% fasting blood sugar (FBS). The marrow plugs were disaggregated, and the dispersed cells were centrifuged and cultured in IMDM with 10% FBS. After 3 days, the nonadherent cells were removed, and medium was replaced every 3–4 days thereafter. Each primary culture was replaced twice to two new culture dishes when the cultures reached about 90% of confluence. When cells were nearly confluent after the second passage, they were harvested and used for coculture experiments.
Neonatal and adult heart cell preparation
Primary neonatal rat cardiomyocytes were isolated from 3-day-old newborn F344 rats as described previously [6]. Briefly, neonatal rats were killed by cervical dislocation and decapitation, and their hearts were removed. Cardiomyocytes were isolated by digestion with 0.5 mg/ml Type II collagenase (Worthington) and 0.5% Trypsin. Cardiomyocytes were purified by preplating (30 min, 37°C), and the cell suspension was plated on 100-mm cell culture dishes at a density of 106 cells with IMDM/10% FBS and antibiotics (100 U/ml penicillin and 10 mg/ml streptomycin solution (Pen/Strep, Gibco Laboratories). The cells were maintained at 37°C in a humidified atmosphere of 5% CO2 until confluence was reached. To check the purity of cultured cardiomyocytes, they were immunohistochemically stained with monoclonal antibody against sarcomeric-α-actinin (Sigma). Adult cardiomyocytes were prepared according to the same method as the above neonatal heart cell isolation. Isolated adult cardiomyocytes were also maintained at 37°C in a humidified atmosphere of 5% CO2.
Coculture system
Mesenchymal stem cells were isolated from adult rat BM as described above and cocultured in four different ways: (1) with neonatal cardiomyocytes, (2) with adult cardiomyocytes, (3) with neonatal cardiomyocytes on the cell culture inserts (double chamber system), and (4) with conditioned medium from neonatal cardiomyocytes. Before direct coculture experiments, MSCs were labeled with 10 μM fluorescent carbocyanine 1,1′-dioctadecyl-1-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) dye (Sigma) for 10 min at room temperature in phosphate-buffered saline (PBS). The DiI-labeled MSCs were plated at a density of 1×105 cells/well in six-well plates containing 4×105 neonatal or adult cardiomyocytes per well. For the double chamber system, MSCs were seeded at a density of 1×105 cells/well in six-well plates, and the cell culture insert containing a total of 4×105 neonatal cardiomyocytes was placed onto a six-well plate. The insert membrane with 0.4 μm pore size was used to avoid direct cell-to-cell interaction between MSCs and neonatal cardiomyocytes instead of allowing for the diffusion of soluble factors between two cell populations. Finally, MSCs were cultured with the conditioned medium recovered from neonatal cardiomyocytes incubated for 7 days in vitro.
Immunofluorescence staining of cells
According to time schedule, passaged cells were plated at a density of 2×104 cells in four-well Lab-Tek Chamber Slides (Nunc) for 24 h. The cells were rinsed twice with PBS, fixed in 4% paraformaldehyde for 15 min at −20°C, and immediately rehydrated with PBS. The slides were blocked with PBS containing 10% fetal bovine serum overnight at 4°C and incubated with either sarcomeric α-actinin, desmin (Sigma), or cardiac myosin heavy chain (Biogenesis). These antibodies were used as described in the original references or the manufacturer’s specifications. After three washes with PBS, the slides were incubated with secondary antibodies of fluorescein isothiocyanate (FITC)-conjugated rabbit antimouse IgG (Sigma), used at a 1:100 dilution, and applied for 1 h at room temperature. Control staining was performed by substituting PBS/10% FBS for the primary antibody. Cell nuclei were stained with 4c6-diamidino-2-phenylindole·2HCl (DAPI) (Sigma) according to the manufacturer’s instructions. After three PBS rinses, the slides were mounted with antifade mounting gel (DAKO) at room temperature, viewed under a phase-fluorescence Carl Zeiss microscope, and photographed.
Fluorescence-activated cell sorting
After 5 days in coculture, DiI-labeled MSCs were stained with an FITC-conjugated antisarcomeric-α-actinin antibody (Sigma). Ten thousands cells were analyzed on a fluorescence-activated cell sorter (FACS) Vantage (Becton Dickinson).
Dye transfer experiment
Neonatal cardiomyocytes were labeled with 2.5 uM of calcein-AM (Calbiochem) for 60 min at 37°C. DiI-labeled MSCs were added to the neonatal cardiomyocytes at a ratio of 1:4. Cells were then cocultured for the times indicated. Dye transfer was measured by flow cytometry in native, unfixed cells by the transfer of calcein to DiI-labeled MSCs. For some experiments, neonatal cardiomyocytes were fixed by 2% paraformaldehyde for 15 min at room temperature, and then these cells cocultured with DiI-labeled MSCs.
Statistical analysis
Data are expressed as mean±SD. Intergroup comparisons were performed using paired Student’s t test. Probability values of P<0.05 were of statistical significance.
Results
MSCs express cardiac-specific markers when directly cocultured with neonatal cardiomyocytes, but not adult cardiomyocytes
When initially plated, MSCs appeared as heterogeneous groups of large flat cells, smaller spindle-shaped cells, and small round cells (Fig. 1a). After 3 days of plating, most of the adherent cells showed typical MSC fibroblastic morphology with large flat and spindle-shaped cells (Fig. 1b). To determine whether MSCs from the adult BM have pluripotency, we examined whether these cells could differentiate into cells other than cardiomyocytes. When treated with osteogenic inducers, some of the MSCs were stained with Alizarin Red S for detection of calcium deposition (Fig. 1c). When MSCs were cultured with adipogenic inducers for 3 weeks, some of the Sca-1+ cells showed cytoplasmic accumulation of oil droplets stained with Oil Red O, indicating that MSCs differentiated into adipocytes (Fig. 1d).
Morphology of MSCs from adult rat bone marrow. When initially plated, at least three distinct cell types are visible: large flat cells (arrowheads), smaller spindle-shaped cells (thin arrows), and small round cells (thick arrows) (a). 3 days after seeding, cultures contain mostly a large, flat, and spindle-shaped cell type (b). Ostegenic and adipogenic differentiation potential of MSCs. Osteogenic differentiation of Sca-1+ cells was induced with ascorbic acid 2-phosphate, dexamethasone, and glycerophosphate for 3 weeks. Alizarin-Red S staining was used for detection of calcium deposition (c). Adipogenic differentiation of Sca-1+ cells was induced by methyl-isobutylxanthine, dexamethasone, indomethacin, and insulin treatment for 3 weeks. Oil-Red O staining showed adipogenic differentiation (d) of Sca-1+ cells
To determine whether neonatal or adult cardiomyocytes affect the differentiation of MSCs into cardiomyocytes, we cocultured MSCs that were labeled with the fluorescent tracer DiI before coculture with neonatal or adult cardiomyocytes to trace the differentiation of MSCs into cardiomyocytes.
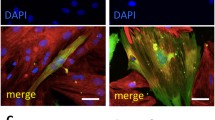
The living DiI-labeling efficiency of MSCs for the coculture experiments was about 50% MSCs by flow cytometry analysis before coculture (data not shown). Immunocytochemical analysis was performed at day 5 of the coculture to examine the expression of cardiac markers such as sarcomeric α-actinin, desmin, and cardiac myosin heavy chain. We observed MSCs that reacted strongly with sarcomeric α-actinin containing well-aligned sarcomere structures in the cytoplasm when cocultured with neonatal cardiomyocytes (Fig. 2a). However, we could not detect any positive signals in the groups of MSCs directly cocultured with adult cardiomyocytes (Fig. 2b), although adult cardiomyocytes used for coculture were mostly stained with sarcomeric α-actinin or cardiac myosin heavy chain. Also, to understand how MSCs respond to paracrine signaling from neonatal cardiomyocytes, the two cell populations were placed in coculture, separated by the membrane that prevented direct cell-to-cell contact, but still allowed paracrine signaling. Figure 2c were the results of MSCs stained with cardiac myosin heavy chain when cultured with the double chamber system. In this case, MSCs located in the bottom did not stain with cardiac markers, although they cultured with neonatal cardiomyocytes. Finally, transdifferentiation of MSCs were not observed when they cultured with conditioned medium from neonatal heart cell culture (Fig. 2d). At any other fields, we could not observe the positive signals. When cultured with double chamber or conditioned medium, MSCs were not labeled with a DiI dye because it was unnecessary to distinguish two types (Fig. 2c,d), showing only a DAPI stain for nuclei. In the group of MSCs cocultured with neonatal cardiomyocyters, desmin and cardiac myosin heavy chain were only detected with a similar result to that of sarcomeric α-actinin, but not cocultured with adult cardiomyocytes or with conditioned medium from neonatal cardiomyocytes (data now shown). Transdifferentiation of MSCs into cardiomyocytes was quantified by flow cytometry analysis (Fig. 3). Initially, DiI-labeled MSCs were detected 2.07±0.40% of positive stain to sarcomeric α-actinin (Fig. 3a). After 5 days of coculture, 9.40±1.15% of MSCs (red channel) expressed sarcomeric-α-actinin, (FITC-labeled, green channel) (Fig. 3b). Moreover, 0.05% of MSCs was observed when they were cultured with conditioned medium from neonatal cardiomyocytes (Fig. 3c).
Expression of sarcomeric α-actinin in MSCs after being cocultured with neonatal cardiomyocytes. MSCs labeled with DiI (a, b) were cocultured with neonatal (a) or adult cardiomyocytes (b), neonatal cardiomyocytes on the cell culture inserts (double chamber system) (c), and conditioned medium from neonatal cardiomyocytes (d) for 5 days. Cells were stained by FITC-conjugated antisarcomeric α-actinin antibody. Cultured MSCs with neonatal cardiomyocytes immunohistochemically stained for sarcomeric-α-actinin (a). DAPI was used for staining nuclei
Quantification of transdifferentiation of MSCs into cardiomyocytes. a, b Representative flow cytometry analyses of MSCs after coculture with neonatal cardiomyocytes (a) or after 5 days of coculture with neonatal cardiomyoyctes (b). Cells were stained with an antisarcomeric-α-actinin antibody (green fluorescence) to identify cardiomyocytes; MSCs are labeled with DiI (red fluorescence). Transdifferentiated MSCs expressing sarcomeric-α-actinin are in upper right quadrant in b. c Quantitative group data. Percentage of MSCs expressing sarcomeric α-actinin at baseline (black bar), after 5 days of coculture with neonatal cardiomyocytes (white bar) or after 5 days of culture with conditioned medium from neonatal cardiomyocytes (gray bar). Values are means±SEM. P<0.05 vs baseline
These results suggest that direct cell-to-cell contact with neonatal, but not adult, cardiomyocytes is necessary for transdifferentiation of MSCs into cardiomyocytes. And the soluble factors by themselves were insufficient to induce differentiation of MSCs into cardiomyocytes.
Gap junctional communication or cell-to-cell contact is involved in transdifferentiation of MSCs into cardiomyocytes but not intercellular fusion
We next investigated whether MSCs connect to neonatal cardiomyocytes by gap junction and calcium-dependent cell-to-cell contact. At first, we incubated the coculture of DiI-labeled MSCs and neonatal cardiomyocytes with the gap junction-permeable fluorescent dye calcein. Quantification by flow cytometry showed that 56.1±2.0% of the DiI-labeled MSCs were positive for calcein after 24 h of coculture (Fig. 4), suggesting neonatal cardiomyocytes are connected to MSCs by gap junctions.
Recent studies reported that BM cells could adopt the phenotype of recipient cells by spontaneous cell fusion. To investigate whether transdifferentiation of MSCs cocultured with neonatal cardiomyocytes is due to cell fusion, we used paraformaldehyde-fixed neonatal cardiomyocytes, which cannot fuse with other cells but have an intact cell surface for coculture. After 5 days of coculture, some MSCs expressed sarcomeric-α-actinin (Fig. 5), indicating that transdifferentiation of MSCs is not due to spontaneous cell fusion. We did not find the difference in the prevalence of differentiation when MSCs cocultured with nonfixed neonatal cardiomyocytes. Taken together, these results suggest that gap junctions or calcium-dependent cell-to-cell communications are necessary for cardiac differentiation of MSCs in vitro.
Discussion
Recent studies demonstrate that the adult heart tissue microenvironment expresses the appropriate signals that allow the exit of these extracardiac cells from their stem cell state and differentiation into myocytes [3–5, 8]. These data suggest that the ability of MSCs to differentiate into cardiomyocytes under the appropriate conditions, as well as the pluripotecy of MSCs, is that they can engraft the heart after implantation. Also, they demonstrate that MSCs are responding to stimuli, such as chemicals or mechanical factors, during the cardiac differentiation period of MSCs. The mechanisms or signal pathways to regulate cardiac differentiation of MSCs are almost unknown because of in vivo nature. However, it is obvious that the microenvironment is an important factor to determine the final phenotypes of MSCs. The elements of microenvironment can be roughly known as chemical or mechanical signals. The chemical signals are cytokines, hormones, and other soluble factors that are produced by either adjacent or distant cells (paracrine factors). The other factor, mechanical signals, may be produced by the effect of cell stretch or shrinkage, the electrical environment of cells, and other local signals.
Therefore, we have established a model to describe cardiac differentiation of MSCs by signals as either mechanical or chemical using various coculture systems: (1) with neonatal cardiomyocytes, (2) with adult cardiomyocytes so simulated MSCs are exposed to various chemical and mechanical signals but different developmental age to influence the activity of cardiac differentiation of MSCs, (3) with neonatal cardiomyocytes on the cell culture inserts to elucidate cell-to-cell interaction effect, thus excluding mechanical signals from adjacent cardiac inducers, or (4) with conditioned medium from neonatal cardiomyocytes to elucidate the effect of unknown soluble growth factors as paracrine signals.
In our coculture study, MSCs appeared to be capable of generating cardiomyocytes when cocultured with neonatal cardiomyocytes, whereas adult cardiomyocytes were not. This suggests that some cardiac inducers in cardiomyocytes may be age-dependent and progressively lost during postnatal life. For example, the cardiac sodium calcium exchanger promoter in adult and neonatal cardiomyocytes was differentially regulated by Nkx2.5 and serum responsive factor [7].
Ijima and collegues [5] recently reported that treatment of nifedipine, an l-type calcium channel antagonist or culture in Ca2+-free media, suppressed contraction of cardiomyocytes and inhibited skeletal muscle cells to express cardiac-specific proteins, indicating that contraction is one of the cardiac inducers. No contraction of adult cardiomyocytes may explain the reason why MSCs were not transdifferentiated into cardiomyocytes when we cocultured with adult cardiomyocytes. But according to previously studies, MSCs can differentiate into cardiomyocytes when injected in normal or acutely injured adult myocardium [3, 10]. Our study cannot exclude the possibility that coculture techniques in this study do not fully demonstrate complex in vivo, and we will make a further study of characterizing isolated adult cardiomyocytes from the adult heart.
According to one of our coculture results, neonatal cardiomyocytes induced MSCs to cardiomyocytes, MSCs did not observe the cardiac differentiation when cultured with neonatal cardiomyocytes using the double chamber system or conditioned medium from neonatal cardiomyocytes. There are some possibilities that unknown soluble factors may not be essential for transdifferentiation of MSCs into cardiomyocytes or exist at low concentrations. These results suggest that direct cell-to-cell contact, but not soluble factors, are important for transdifferentiation of MSCs into cardiomyocytes.
To be functional characteristics of cardiac differentiation of MSCs, they must become electrically coupled to neighboring cardiomyocytes, which is typically established by gap junctions [5, 6]. In our coculture, MSCs showed gap junctional communication with adjacent-beating neonatal cardiomyocytes, as evidenced by dye transfer experiments.
Recent reports suggested that cell fusion may play a role for transdifferentiation [8]. In detail, mouse BM cells can fuse spontaneously with embryonic stem cells in culture, and spontaneously fused BM cells can subsequently adopt the phenotype of the recipient cells. In other words, the altered phenotype of the embryonic stem cell does not arise from a direct conversion of the cell type, but rather from cell fusion. On the contrary, some studies have reported a high level of transdifferentiation capable of excluding the possibility of cell fusion [4, 9]. In our study, the transdifferentiation rate (net values are about 7%) was much higher than the frequency of spontaneous cell fusion [8] (2–11 clones per 106 BM cells). We found cardiomyocytes transdifferentiated from MSCs, although cocultured with paraformaldehyde-fixed, dead neonatal cardiomyocytes. It demonstrates that MSCs can be differentiated into cardiomyocytes in our coculture condition, although some portion of transdifferentiated cardiomyocytes can be derived by cell fusion.
In conclusion, MSCs can differentiate into cardiomyocytes by direct cell-to-cell interaction with neonatal cardiomyocytes but not adult cardiomyocytes or secreted factors from neonatal cardiomyocytes. This coculture system may be useful for simulating the cardiac environment and evaluating phenotypic and functional parameters in vitro and, furthermore, help various stem cell researches.
References
Makino S, Fukuda K, Miyoshi S et al (1999) Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 103:697–705
Lough J, Barron M, Brogley M et al (1996) Combined BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev Biol 178:198–202
Wang J-S, Shum-Tim D, Galipeau J et al (2000) Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg 120:999–1006
Badorff C, Brandes RP, Popp R et al (2003) Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation 107:1024–1032
Iijima Y, Nagai T, Mizukami M et al (2003) Beating is necessary for transdifferentiation of skeletal muscle-derived cells into cardiomyocytes. FASEB J 17:1361–1363
Fukuhara S, Tomita S, Yamashiro S et al (2003) Direct cell–cell interaction of cardiomyocytes is key for bone marrow stromal cells to go into cardiac lineage in vitro. J Thorac Cardiovasc Surg 125:1470–1480
Muller JG, Thompson JT, Edmonson AM et al (2002) Differential regulation of the cardiac sodium calcium exchanger promoter in adult and neonatal cardiomyocytes by Nkx2.5 and serum response factor. J Mol Cell Cardiol 34:807–821
Terada N, Hamazaki T, Oka M et al (2002) Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416:542–545
Lagasse E, Connors H, Al-Dhalimy M et al (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6:1229–1234
Shake JG, Gruber PJ, Baumgartner WA et al (2002) Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects, Ann Thorac Surg 73:1919–1925
Acknowledgements
This work was supported by the Brain Korea 21 project and the Korea Health 21 R&D project, Ministry of Health and Welfare, Republic of Korea (Dr. Lim, 01-PJ10-PG8-01EC01-0027).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, J., Shim, W.J., Ro, Y.M. et al. Transdifferentiation of mesenchymal stem cells into cardiomyocytes by direct cell-to-cell contact with neonatal cardiomyocyte but not adult cardiomyocytes. Ann Hematol 84, 715–721 (2005). https://doi.org/10.1007/s00277-005-1068-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-005-1068-7