Abstract
A case of disseminated infection with Fusarium oxysporum following chemotherapy of acute myelogenous leukemia is reported. Antifungal treatment was successful with a 13-day course of oral terbinafine 250 mg t.i.d. in combination with amphotericin B deoxycholate 1.0–1.5 mg/kg qd and subsequently intravenous liposomal amphotericin B 5 mg/kg qd. Preceding monotherapy with amphotericin B deoxycholate 1.0–1.5 mg/kg qd had not stopped the progression of infection. The combination therapy described here represents a novel approach to the treatment of Fusarium spp. in the immunocompromised host in whom Fusarium spp. are known to cause disseminated infection with high mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case report
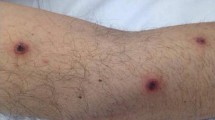
We report on a 59-year-old male patient with acute myeloblastic leukemia secondary to myelodysplastic syndrome. The patient received a first induction chemotherapy with thioguanine, cytarabine, and daunorubicin followed by a second induction chemotherapy with high-dose cytarabine and mitoxantrone resulting in an expected overall neutropenia (<500 neutrophils/µl) of 6 weeks’ duration. Complete remission was achieved after the first course and the second course was administered in time according to the protocol while the patient was still neutropenic. On the 20th day of neutropenia the patient developed fever of 38.6°C and was treated with ceftriaxone 2 g and gentamicin 5 mg/kg once daily. Six days later, an oval dark-red and itching papulous skin lesion of 5 mm diameter appeared on the right foot (Fig. 1, left). Because of persistent fever and metastatic spreading of the skin lesions, therapy was changed to meropenem 1 g t.i.d., vancomycin 1 g b.i.d., and amphotericin B deoxycholate 1 mg/kg qd.
The sequence of anti-infective agents administered in relation to clinical symptoms is given in Table 1. Over the next 3 days, several blood cultures were positive for molds that were morphologically suggestive of Acremonium or Fusarium species. Pulmonary infiltrates were ruled out by multislice computed tomography. Despite antifungal treatment, the patient did not defervesce. Physical exams revealed numerous additional skin lesions with central necrosis (Fig. 1, right). Nearly 25% of the patient’s skin was covered by these lesions. Septic vasculitis on the basis of a mold fungemia was verified histologically and by culture (Fig. 2). The skin lesions were extremely painful requiring continuous intravenous morphine infusions. The patient’s condition deteriorated despite increase of the amphotericin B dose to 1.5 mg/kg qd. On the 35th day of neutropenia, i.e., 9 days after the occurrence of the first skin lesion, preliminary in vitro antifungal susceptibility testing suggested rather low susceptibility to amphotericin B, but possible susceptibility to terbinafine. Therefore, oral terbinafine 250 mg t.i.d. was added to the treatment regimen.
After 3 days of antifungal combination therapy, the patient developed septic shock complicated by acute renal and pulmonary failure. Amphotericin B deoxycholate was replaced by liposomal amphotericin B 5 mg/kg qd IV. The patient required intermittent dialysis and respiratory ventilation over a period of 6 weeks. Progression of the skin lesions stopped after 1 week of antifungal combination therapy. Due to acute hepatotoxicity (bilirubin 10 mg/dl) antifungal treatment was stopped after 13 days of combination therapy. However, the skin lesions further improved and completely resolved with neutrophil reconstitution above 1000/µl.
The molds were identified morphologically as Fusarium oxysporum (Fig. 3). The results of in vitro susceptibility testing are given in Table 2. Terbinafine, administered by mouth and by nasogastric tube, achieved maximal plasma concentrations of 0.433 µg/ml (Fig. 4).
The acute myelogenous leukemia relapsed after 3 months of complete remission. The patient then received allogeneic bone marrow transplantation from a matched unrelated donor and was discharged 2 months later without secondary antifungal prophylaxis being given. Two months after discharge, the patient was readmitted with severe graft-versus-host reaction, developed a fever, and subsequently died of cerebral hemorrhage. Blood cultures were again positive for Fusarium oxysporum suggesting a reoccurrence of fungal infection. However, these results were communicated only after the patient’s death.
Discussion
We describe the remission of a disseminated Fusarium oxysporum infection refractory to amphotericin B deoxycholate monotherapy. Subsequent antifungal combination therapy with liposomal amphotericin B and terbinafine was successful.
Fusarium spp. are ubiquitous and may be found on rice, beans, soybeans, and grains as saprophytes or as plant pathogens, as well as in soil specimens. They have also been found in potted plants, and hospital water supply systems have been discussed as a reservoir [1]. Fusarium spp. have been reported as pathogens in the immunocompromised host before [2].
Fusarium spp. can cause disseminated fungal infection, but most often present as localized infection such as onychomycosis. In patients with leukemia, sinusitis as well as pneumonia and fungemia have been observed. Skin lesions are documented frequently in hematogenously disseminated infection [2]. In the setting of hemato-oncology, these skin lesions mostly occur during neutropenia or the immediate post-transplantation phase. After solid organ transplantation, fusariosis tends to be rather localized in contrast to the more disseminated forms in patients with hematological malignancies or after bone marrow transplantation [3]. In the latter, antifungal treatment is often unsuccessful [2].
The macroscopic and microscopic exams are decisive for the diagnosis of fusariosis. Molecular methods of identification such as 28S rRNA sequencing may then be helpful to differentiate between different species [4].
In this patient, skin lesions were first noticed on the 26th day of neutropenia. Initially, gram-positive infection was considered to be the most likely cause. However, similar lesions may also be caused by Candida spp., Aspergillus spp., and Mucorales as well as P. aeruginosa in ecthyma gangrenosum. In this patient, molds were detected both in blood cultures and in skin biopsy. During treatment with amphotericin B deoxycholate, nephrotoxicity occurred and liposomal amphotericin B had to be administered instead.
Fusarium isolates show in vitro and in vivo resistance to conventional antifungal agents, thus representing a therapeutic challenge. Fusarium oxysporum frequently shows reduced susceptibility to amphotericin B [2, 5, 6, 7, 8, 9, 10]. In this patient, amphotericin B monotherapy was clinically ineffective, despite reports indicating a response of fusariosis to conventional or lipid-based amphotericin B. As in this patient, lipid-based amphotericin B was administered when nephrotoxicity occurred under treatment with amphotericin B deoxycholate [11]. In vitro testing of itraconazole suggested ineffectiveness, too. For 5-flucytosine, ketoconazole, miconazole, fluconazole, and itraconazole high MIC levels have been described elsewhere, and primary resistance to caspofungin and other ß-1,3-glucan-synthesis inhibitors has been reported [5, 6, 9, 10, 12, 13, 14]. In data presented to the Food and Drug Administration (FDA), 9 (43%) of 21 patients with fusariosis had a complete or partial response to voriconazole [15]. Meanwhile voriconazole has been approved for the treatment of fusariosis, but was not yet available at the time when this patient required treatment. Terbinafine is an allylamine inhibiting squalene epoxidase, which interferes with the fungal cell membrane synthesis. However, terbinafine is not approved for treatment of invasive fungal infection. Terbinafine minimal inhibitory concentration (MIC) values against Fusarium spp. have been reported in the range of 0.25 to >128 µg/ml; thus, its benefit must be evaluated on a case-by-case basis [7, 16, 17].
Despite the lack of effectiveness in monotherapy, the combination of caspofungin and amphotericin B seemed to be efficacious in vitro in some isolates [10]. In general, combination therapies may be discussed especially in refractory fungal disease, but the pattern of infection (localized versus disseminated) and pharmacokinetic parameters have to be considered. In the patient we report on, a disseminated Fusarium oxysporum infection refractory to amphotericin B deoxycholate was successfully treated with combination therapy of liposomal amphotericin B and terbinafine. However, complete eradication of fungal infection cannot be assumed, as Fusarium oxysporum was isolated again during a subsequent and eventually fatal episode of severe immunosuppression.
References
Anaissie EJ, Kuchar RT, Rex JH, Francesconi A, Kasai M, Muller FM, Lozano-Chiu M, Summerbell RC, Dignani MC, Chanock SJ, Walsh TJ (2001) Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: a new paradigm for the epidemiology of opportunistic mold infections. Clin Infect Dis 33:1871–1878
Boutati EI, Anaissie EJ (1997) Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood 90:999–1008
Walsh TJ, Groll AH (1999) Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl Infect Dis 1:247–261
Hennequin C, Abachin E, Symoens F, Lavarde V, Reboux G, Nolard N, Berche P (1999) Identification of Fusarium species involved in human infections by 28S rRNA gene sequencing. J Clin Microbiol 37:3586–3589
Guarro J, Gene J (1995) Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis 14:741–754
Pujol I, Guarro J, Gene J, Sala J (1997) In-vitro antifungal susceptibility of clinical and environmental Fusarium spp. strains. J Antimicrob Chemother 39:163–167
Speeleveld E, Gordts B, Van Landuyt HW, De Vroey C, Raes-Wuytack C (1996) Susceptibility of clinical isolates of Fusarium to antifungal drugs. Mycoses 39:37–40
Warnock DW (1998) Fungal infections in neutropenia: current problems and chemotherapeutic control. J Antimicrob Chemother 41:95–105
Espinel-Ingroff A (1998) In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol 36:198–202
Arikan S, Lozano-Chiu M, Paetznick V, Rex JH (2002) In vitro synergy of caspofungin and amphotericin B against Aspergillus and Fusarium spp. Antimicrob Agents Chemother 46:245–247
Letscher-Bru V, Campos F, Waller J, Randriamahazaka R, Candolfi E, Herbrecht R (2002) Successful outcome of treatment of a disseminated infection due to Fusarium dimerum in a leukemia patient. J Clin Microbiol 40:1100–1102
Espinel-Ingroff A (1998) Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol 36:2950–2956
Pfaller MA, Marco F, Messer SA, Jones RN (1998) In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis 30:251–255
Del Poeta M, Schell WA, Perfect JR (1997) In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother 41:1835–1836
Johnson LB, Kauffman CA (2003) Voriconazole: a new triazole antifungal agent. Clin Infect Dis 36:630–637
Ryder NS (1999) Activity of terbinafine against serious fungal pathogens. Mycoses 42 [Suppl 2]:115–119
De Pauw BE (2000) New antifungal agents and preparations. Int J Antimicrob Agents 16:147–150
Acknowledgements
The authors thank Gottfried Weidinger MD, Novartis Pharma, 90429 Erlangen, Germany and Jannick Denouël MD, Novartis Pharma SA, 92500 Rueil-Malmaison, France for the determination of terbinafine plasma concentrations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rothe, A., Seibold, M., Hoppe, T. et al. Combination therapy of disseminated Fusarium oxysporum infection with terbinafine and amphotericin B. Ann Hematol 83, 394–397 (2004). https://doi.org/10.1007/s00277-003-0795-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-003-0795-x