Abstract
A 57-year-old man with acute myeloid leukemia (AML) French-American-British (FAB) 4 developed disseminated invasive cerebral and pulmonary aspergillosis during postinduction aplasia. According to international consensus, infection was categorized as probable (two host factors: deep neutropenia for >10 days and refractory fever for >96 h; major clinical criteria of lower respiratory tract and CNS invasive fungal infection; positive results for galactomannan antigen in three blood samples). After the failure of standard amphotericin-based therapy, the spectacular regression of multifocal brain and lung lesions was rapidly achieved under a caspofungin acetate/voriconazole combination. Further permanent caspofungin maintenance with voriconazole added during aplasia periods permitted two consolidation courses and autograft-based intensification without any delay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The management of disseminated invasive aspergillosis (IA) in highly immunocompromised hematological patients remains a major problem. Aspergillosis has become an increasingly prevalent infection with the rise of immunosuppression and its incidence ranges from 5 to more than 20% in high-risk groups [1]. The lungs are the most common site involved (87%) followed by sinuses/nose (16%) and the brain (8%) [2]. Despite improvements in diagnosis and the advent of newer formulations of amphotericin B (AmB) the mortality of IA still ranges from 50 to over 90% [3, 4]. Because of the extremely poor prognosis of IA and toxicity of AmB preparations, newer antifungal drugs and drug combinations with improved efficacy and reduced toxicity are needed. Itraconazole alone has a good safety and tolerability profile and was shown to be effective against Aspergillus [5], but polyene/azole combinations are not recommended for treatment of invasive fungal infections for concerns of their antagonism in vivo (Infectious Diseases Society of America guidelines) [6]. Recently, two new agents, triazole voriconazole (VOR) and echinocandin caspofungin acetate (CAS), were reported to possess powerful activity against IA [7, 8]. Because of different mechanisms of action and absence of cross-toxicity the CAS/VOR combination could be a promising challenge for enhancing the efficacy of each other. Laboratory findings [9] and animal models studies [10] confirmed a synergic interaction between these two agents. The application of the CAS/VOR combination in our patient led to a rapid resolution of disseminated IA and supports the hypothesis of its synergic antifungal activity without any additional toxicity.
Case report
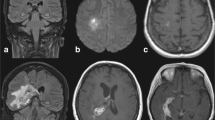
A 57-year-old Caucasian man with no significant medical history was admitted to our department for hyperleukocytosis (180×109/l), anemia (9 g/dl), and thrombocytopenia (45×109/l). The diagnosis of acute myeloid leukemia (AML) French-American-British (FAB) M4 with (inv16) was made. On admission, there was no evidence for hepatosplenomegaly or neurological manifestations, and the chest X-ray and lumbar puncture were normal. On day 8 of induction therapy [daunorubicin 60 mg/m2 days 1–3; continuous cytarabine (AraC) 200 mg/m2 days 1–7], the patient developed fever and hypoxia, and the chest X-ray showed pulmonary infiltrates. Treatment with broad-spectrum antibiotics was started immediately. Intravenous AmB (Fungizone, Bristol-Myers-Squibb, Princeton, N.J., USA, 1 mg/kg per day) was added on day 13 for persistent fever, worsening of clinical conditions, and radiological signs of multiple bilateral pulmonary infiltrates with halo signs on chest CT scan (Fig. 1A). Two days thereafter (day 15), the patient became withdrawn and confused. The CT scan of the brain revealed five nodular lesions with perifocal edema in the frontal and parietal lobes (Fig. 1B). Highly positive results of Aspergillus galactomannan antigen (GA) were found in a sandwich enzyme-linked immunosorbent assay (ELISA) in three consecutive peripheral blood samples. The cerebrospinal fluid (CSF) was normal with no atypical cells or hyperproteinorrhachia; it was also negative for GA, Cryptococcus neoformans antigen, Toxoplasma gondii polymerase chain reaction (PCR), and standard cultures including Nocardia. Since stereotactic or open surgery was not possible because of the patient’s general condition, cerebral and pulmonary lesions were classified as probable disseminated aspergillosis [11]. On day 18 CAS (Cansidas, Merck, Sharp, & Dohme, Lyon, France, 70 mg on day 1 followed by 50 mg daily) was added to AmB. After 6 days of combination AmB/CAS therapy, the patient’s respiratory and neurological status continued to worsen and the CT scan on day 23 showed aggravation of pulmonary lesions with appearance of cavities within areas of consolidation. For this reason AmB was switched to intravenous voriconazole (Vfend, Pfizer, New York, USA, 6 mg/kg b.d. the 1st day and then 4 mg/kg b.d.). After 6 days of CAS/VOR therapy and along with recovery from aplasia, the patient’s clinical condition improved with disappearance of fever and resolution of pulmonary and neurological clinical manifestations. The complete remission was established and on day 37 the patient was discharged on CAS monotherapy (50 mg/day). The CT scan on day 46 of induction (day 28 of CAS) revealed partial regression of cerebral lesions with a 44% regression of pulmonary nodules. Immediately after, the first consolidation course was performed and the aplasia period was covered by addition of VOR (13 i.v. 4 mg/kg doses) to the CAS. On day 72 (day 54 of CAS), the pulmonary lesions largely regressed with nearly complete disappearance of cerebral lesions (Fig. 2A, B). It permitted full high-dose AraC-based superconsolidation. The same dose of VOR was added again to cover the neutropenic period. The CT scan of the brain and chest on day 78 showed no evidence of cerebral or pulmonary lesions. After the end of post-superconsolidation aplasia (day 94), CAS was definitely stopped (the patient received 76 days of CAS in total). Single VOR prophylaxis (200 mg/day p.o.) was continued through auto-PBSCT (day 0=day 119) and stopped on day 239 from diagnosis. Currently, our patient is doing well in first complete remission 10 months after diagnosis.
Discussion
The case fatality rate of 86.7% for BMT recipients and 88.1% for patients with CNS or disseminated aspergillosis confirms that this complication remains a highly lethal opportunistic infection [3]. Prompt administration of effective antifungal therapy and early initiation of active salvage therapy in the case of refractory fungal disease is crucial to secure the control of infection [12, 13]. High-dose (8–10 mg/kg) liposomal AmB was reported to penetrate into the CSF [14] in animal models and was successfully used in leukemia patients with cerebral aspergillosis [15], but this option was not widely tested because of its high cost. Despite the introduction of lipid formulations of AmB, about 50% of patients with aspergillosis will fail primary therapy [16]. The new azole voriconazole was shown to be superior to AmB in terms of efficacy and survival and actually approved for the first-line treatment of IA [17]. CAS represents a new class of echinocandins with a novel mechanism of action different from that of AmB or azoles. It inhibits the synthesis of β-D-glucan in fungal cell wall causing a rapid fungicidal activity in Candida and an inhibitory effect on most Aspergillus spp. [18]. CAS was shown to be effective for the treatment of IA in patients who are refractory or intolerant to previous antifungal treatment and could be considered as salvage therapy [19]. As the new formulations act in different ways on the integrity of the fungal cell, it is logical to suppose the presence of a synergistic fungicidal effect of its combinations. Perea et al. [9] in vitro and Kirkpatrick et al. [10] in animal models with brain aspergillosis demonstrated a possible synergic or additive effect of the CAS/VOR combination. One clinical case of successful treatment of disseminated IA by CAS/VOR combination in a leukemic child was reported recently by Gatbois et al. [20]
According to international consensus [11], our patient met the criteria of probable disseminated invasive pulmonary and cerebral aspergillosis. Six-day first-line AmB therapy did not improve the clinical and radiological status of the patient. Even if there is no accurate pharmacokinetic data on the bioavailability of CAS and VOR in the CSF, we chose CAS as the second-line agent following the report of favorable response in two of eight refractory cases of Aspergillus brain abscesses treated with this drug [19]. After 6 days of AmB/CAS treatment, the results were unsatisfactory and the switch to CAS/VOR was based on two case reports of successful treatment of brain aspergillosis [21] and Scedosporium [22] abscesses with voriconazole. The clinical brain and pulmonary recovery was favorable and dramatically rapid. Despite two further consolidation courses with repeated periods of neutropenia, there was direct evidence of fast regression of cerebral and pulmonary lesions. There is no direct proof that the cure was exclusively due to combination. Parallel resolution of neutropenia and complete remission of AML undoubtedly played a role. Because of the rarity of favorable outcomes in equivalent situations, the optimal drug combination and treatment duration are not well established. In our case, the absence of any hepatic or renal toxicity allowed us to prolong CAS maintenance for 76 days and to add VOR to cover each period of aplasia. This is the first case of successful treatment of disseminated cerebral and pulmonary IA in an adult patient with a CAS/VOR combination. The fast positive response confirms the hypotheses of good brain penetration of these drugs and their possible synergic/addictive antifungal activity.
References
Denning DW (1998) Invasive aspergillosis. Clin Infect Dis 26:781–803, quiz 804–805
Denning DW, Marinus A, Cohen J, et al. (1998) An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC Invasive Fungal Infections Cooperative Group. J Infect 37:173–180
Lin SJ, Schranz J, Teutsch SM (2001) Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 32:358–366
Patterson TF, Kirkpatrick WR, White M, et al. (2000) Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 79:250–260
Andriole VT (2000) Current and future antifungal therapy: new targets for antifungal therapy. Int J Antimicrob Agents 16:317–321
IDSA (2000) Practice guidelines for diseases caused by aspergillus. Clin Infect Dis 30:696–709
Herbrecht R, Denning DW, Patterson TF, et al. (2002) Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415
Pacetti SA, Gelone SP (2003) Caspofungin acetate for treatment of invasive fungal infections. Ann Pharmacother 37:90–98
Perea S, Gonzalez G, Fothergill AW, Kirkpatrick WR, Rinaldi MG, Patterson TF (2002) In vitro interaction of caspofungin acetate with voriconazole against clinical isolates of Aspergillus spp. Antimicrob Agents Chemother 46:3039–3041
Kirkpatrick WR, Perea S, Coco BJ, Patterson TF (2002) Efficacy of caspofungin alone and in combination with voriconazole in a Guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother 46:2564–2568
Ascioglu S, Rex JH, de Pauw B, et al. (2002) Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34:7–14
Caillot D, Casasnovas O, Bernard A, et al. (1997) Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol 15:139–147
Barnes AJ, Oppenheim BA, Chang J, et al. (1999) Early investigation and initiation of therapy for invasive pulmonary aspergillosis in leukaemic and bone marrow transplant patients. Mycoses 42:403–408
Adler-Moore JP, Chiang SM, Satorius A, et al. (1991) Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome). J Antimicrob Chemother 28 [Suppl B]:63–71
Mahlknecht U, von Lintig F, Mertelsmann R, et al. (1997) Successful treatment of disseminated central nervous aspergillosis in a patient with acute myeloblastic leukemia. Leuk Lymphoma 27:191–194
Denning DW (1996) Therapeutic outcome in invasive aspergillosis. Clin Infect Dis 23:608–615
Herbrecht R, Denning DW, Patterson TF, et al. (2002) Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415
Denning DW (2002) Echinocandins: a new class of antifungal. J Antimicrob Chemother 49:889–891
Maertens J, Raad I, Sable CA, et al. (2000) Multicenter noncomparative study to evaluate safety and efficacy of caspofungin in adults with invasive aspergillosis refractory or intolerant to amphotericin B, amB lipid formulations or azoles (abstract 1103:371). 40th ICAAC, Toronto, Ontario, Canada, 17–20 September 2000
Gatbois E, Adjaoud D, Larroquet M, et al. (2002) Successful treatment of disseminated aspergillosis by caspofungin and voriconazole in a leukemic child. J Mycol Med 12:90–92
Schwartz S, Milatovic D, Thiel E (1997) Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute leukaemia. Br J Haematol 97:663–665
Nesky MA, McDougal EC, Peacock JE Jr (2000) Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin Infect Dis 31:673–677
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Damaj, G., Ivanov, V., Le Brigand, B. et al. Rapid improvement of disseminated aspergillosis with caspofungin/voriconazole combination in an adult leukemic patient. Ann Hematol 83, 390–393 (2004). https://doi.org/10.1007/s00277-003-0792-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-003-0792-0