Abstract
The main purpose of this report is to focus on the importance of an accurate etiologic diagnosis of gastrointestinal complications during chemotherapy for acute myeloid leukemia, taking into account that a syndrome characterized by bowel wall thickening associated with diarrhea and abdominal pain may have etiologies different from neutropenic enterocolitis (NE) and in such a case necessitate a different treatment approach. We describe a case of a 46-year-old woman affected by acute myeloid leukemia presenting the onset of a syndrome with clinical features of NE. Supportive therapy for NE was instituted, but during treatment the patient presented a life-threatening gastrointestinal bleeding and was submitted in emergency to hemicolectomy. Following surgery, the patient recovered completely and she is currently alive in complete remission after receiving allogeneic bone marrow transplantation. Histological examination of the surgical specimens showed that the acute abdominal syndrome was related to massive infiltration of the bowel by leukemia cells. A correct baseline evaluation and a prompt diagnosis of the complication may help in making the therapeutic decision, which in our case led necessarily to a surgical procedure, because the bleeding was due to post-chemotherapy necrosis of the leukemic infiltrating tissue. A close collaboration between the hematologist and the surgeon may provide guidelines for behavior in such cases, giving these patients the possibility of survival and the opportunity to carry on the treatment planned for the primary disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neutropenic enterocolitis (NE) is a frequent complication of intensive chemotherapy in acute myeloid leukemia patients. Clinical symptoms are usually fever, diarrhea, and abdominal pain, associated with sonographic detection of pathologic bowel wall thickening. The mortality rate is high, especially in patients presenting a mural thickness of more than 10 mm, and in most cases microbiologic findings on pathologic specimens show Gram− bacterial contamination or fungal infection [1, 2, 3]. We describe a case of acute myeloid leukemia presenting the early onset of a syndrome with the clinical features of NE, but related to massive infiltration of the bowel by leukemia cells.
Case report
In April 2001, a 46-year-old woman presented with a 1-month history of fever, weakness, and gum hypertrophy, already treated with antibiotic therapy by the family physician. The peripheral blood count at admittance showed: hemoglobin (Hb): 5.9 g/dl, WBC: 4.5×109/l, and platelets: 74×109/l. The peripheral blood differential count showed: 7% polymorphonuclear neutrophils (PMN), 78% monocytes, 8% lymphocytes, and 7% blasts.
Physical examination demonstrated only the presence of inguinal adenopathies, and chest X-ray and abdominal ultrasonography scan (US) were negative. The diagnosis of acute myeloid leukemia (AML) was confirmed by a bone marrow aspirate, which presented an almost total infiltration by monocytoid blasts, with the following features at cytochemical staining: myeloperoxidase negative, 27% Sudan positive, 75% α-naphthyl-acetate positive, with 40% inhibition by NAF [AML type M5 according to French-American-British (FAB) classification]. Immunophenotypic evaluation by flow cytometry showed that the blast cells reacted with antibodies to CD45, HLA-DR, CD13, CD33, CD15, CD56, and CD14. Molecular analysis on the bone marrow showed the absence of the most commonly tested rearrangements (AML1/ETO, CBFβ/MYHII, BCR/ABL, PML/RARα, MLL), while cytogenetics were not evaluable because of absence of metaphases. No CNS involvement was detectable by lumbar puncture.
Induction treatment started on 24 April 2001, consisting of hydroxyurea 2 g/m2 per day for 5 days, followed by polychemotherapy with cytarabine 100 mg/m2 per day by continuous infusion on days 1–10, etoposide 100 mg/m2 on days 1–5, and daunorubicin 50 mg/m2 on days 1, 3, and 5. Prophylactic treatment with oral quinolones and amphotericin B was started at the time of admission in consideration of profound granulocytopenia.
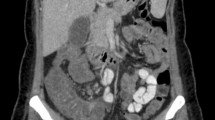
On day 5 of chemotherapy the patient presented fever >38.5°C, abdominal pain localized in the lower quadrants, and diarrhea (>four stools daily); the sonographic scan documented a slightly abnormal thickening of the bowel wall (6 mm), in particular of the left colic flexure.
Broad-spectrum antibiotic therapy with ceftriaxone and amikacin was promptly instituted, and total parenteral nutrition was started the same day, but 48 h after treatment the clinical condition worsened, abdominal pain was extended to all quadrants while the fever and diarrhea persisted. Ultrasonographic monitoring on day 10 (last day of chemotherapy), on day 16, and on day 21 documented a progressive increase in bowel wall thickness of the whole colic tract and part of the ileum, which appeared distended and fluid-filled, with a maximum thickness of 16 mm.
Owing to suspicion of neutropenic enterocolitis, antibiotic therapy was initially intensified by adding fluconazole and metronidazole and substituting ceftriaxone with meropenem. Subsequently, on day +16, i.v. amphotericin B was started, but no improvement was observed in the fever or in the abdominal symptoms.
On day +21 the patient's condition was very serious. She was persistently granulocytopenic and thrombocytopenic, although daily platelet transfusions from pooled donors and from a single donor were given. The fever was refractory to acetaminophen and noramidopyrine, and she also presented a coagulopathy requiring plasma infusions and initial signs of fluid overload with pulmonary subedema.
A bone marrow aspirate performed the same day showed initial granulopoietic reconstitution. Thus, we decided to start granulocyte colony-stimulating factor (G-CSF) 5 µg/kg, which was administered for 3 days. Within 48 h of G-CSF administration, the neutrophil value reached 1×109/l, fever persisted, but abdominal distension and diarrhea progressively improved. Meanwhile the patient received plasma, albumin infusions, furosemide, and O2 therapy to treat fluid overload.
On day +30 in the morning the patient was getting better: the fever had disappeared and O2 therapy was no longer necessary. She was still on meropenem, metronidazole, and amphotericin B, and the blood cell count showed Hb 11.8 g/dl, WBC 13.7×109/l (PMN 12.4×109/l), and platelets 56×109/l independent from transfusion. In the afternoon rectal bleeding started, getting worse in the following hours, and she lost about 4 g hemoglobin within 24 h.
Treatment with i.v. somatostatin and omeprazole was promptly instituted and the patient received eight packed RBC units during the first 24 h. Arteriography of the upper/lower mesenteric district and celiac tripod was not able to detect the source of the bleeding, and although her general condition improved slightly in the following days, occasional bleeding episodes persisted.
Seven days after the first episode bleeding dramatically worsened, causing hemorrhagic shock refractory to supportive transfusional measures. The patient was then transferred to the emergency surgical department where she was submitted to hemicolectomy with end-to-end anastomosis of the ileum with descending colon.
Ten days after surgery, the patient was discharged from hospital, in good general condition, oral nutrition, and normally canalized. Two months thereafter she received consolidation chemotherapy followed by allogeneic bone marrow transplantation, and currently she is alive in continuous complete remission.
Pathologic features
Gross pathology
The surgical specimen consisted of a 90-cm-long subtotal colectomy. The bowel appeared shortened because of the edema, which diffusely involved the subserosal connective tissue and mucosa. The serosa was covered by a delicate and fibrinous layer and by a few hemorrhagic clots. The mucosa was completely effaced by numerous superficial erosions and by deep ulcerative lesions, which were diffusely distributed and replete with green colored necrotic tissue. These ulcerative lesions were irregularly round or linear shaped; moreover, they were mixed with numerous and scattered polypoid formations ranging from 0.2 to 1 cm in size.
Microscopy
We could distinguish in the mucosa three types of lesions:
-
1.
Normal appearing mucosa and polypoid formations outlined by well-preserved superficial epithelium, which displayed a lamina propria involved by focal and diffuse monomorphic infiltrate of 15–25-μm-sized neoplastic cells admixed to a number of plasma cells and a few lymphocytes. This neoplastic tissue effaced and pushed off the glandular structures (Fig. 1). It is worth noting that neoplastic cells could be distinguished in large and medium cells. The large ones were characterized by atypical and multilobated nuclei and by evident and vacuolated cytoplasms; they were characterized by a strong cytoplasmic immunoreactivity for CD68-specific monoclonal antibodies (MoAbs). The small cells displayed a kidney-shaped nucleus and a thin basophilic cytoplasm with few vacuoles resembling monocytes. These cells were immunoreactive for CD68- and CD15-specific MoAbs.
-
2.
Adjacent to the non-ulcerative lesions, we could observe areas of the lamina propria involved by edema, dystrophic appearance of fibroblasts, foci of fibrinoid necrosis, and atrophy of the overlayered superficial epithelium. These dystrophic areas were also surrounded by a diffuse proliferation of large monocytoid neoplastic cells immunoreactive for CD68 and CD15 MoAbs.
-
3.
The ulcerative lesions displayed an aphthoid aspect. They consisted of a deep knife-like fissure containing necrotic tissue surrounded by a diffuse proliferation of monocytoid neoplastic cells immunoreactive for CD68 and CD15 MoAbs (Fig. 2).
All these types of lesions were irregularly surrounded by a fibroblastic hyperplasia and by a few inflammatory cells predominantly represented by polytypic plasma cells and CD3-positive lymphocytes. It is worth noting that these neoplastic infiltrates displayed a frequent transmural distribution, thus determining the acute neoplastic serosal reaction. PAS staining was not able to detect either microorganism invasion of the mucosa or intravascular fungal spread.
Discussion
NE is a clinical syndrome that most frequently occurs after a median of 12 days from start of chemotherapy. A recent study by Cartoni et al. on 1450 leukemia patients reports a 6% incidence of NE and suggests a possible correlation between the degree of bowel wall thickening detected by ultrasonography and the outcome of patients. In particular, 60% of patients with mural thickness greater than 10 mm died from this complication [4].
In our experience we observed the case of a 46-year-old female presenting a very early onset of clinical symptoms suggestive of NE (5th day of chemotherapy), with a progressive increase of mural thickness until 16 mm. This syndrome was refractory to intensive supportive treatment consisting of antibiotic and antimycotic therapy and total parenteral nutrition; it became life threatening on day 37 of chemotherapy when a surgical operation was necessary. Analysis of the surgical specimen documented a leukemic infiltration of the bowel.
The case suggests some considerations, both from a diagnostic and therapeutic point of view. First of all, not all clinical syndromes characterized by bowel wall thickening, diarrhea, and bleeding must necessarily be considered as NE. The early onset, about 4–5 days from the start of chemotherapy, should alert the physician to a non-infective pathogenesis, especially if the patient is affected by a M4-M5 subtype, which is often associated with extramedullary infiltration [5, 6, 7, 8, 9]. Unfortunately, even an accurate baseline ultrasonographic evaluation of the bowel does not seem to be able to detect cases of abnormal wall thickening at diagnosis, and a routine endoscopic monitoring is not feasible in these patients. There are some data in the literature concerning the use of magnetic resonance imaging to explore neoplastic bowel infiltration, but prospective studies are lacking [10].
A possible explanation of the US picture in this case may be the inflammatory reaction and edema following the transmural necrosis due to chemotherapy. Concerning the different aspect of the lesions described in the surgical specimen, they probably represent different evolutive stages of the mucosal damage.
In conclusion, the collaboration between the hematologist, the radiologist, and the surgeon may increase the probability of survival of these patients by a close monitoring in the acute phase and a timely intervention if the surgical approach becomes undelayable. This allows not only the recovery from the acute episode, but also the possibility to complete the treatment planned including a transplant procedure.
References
Alt B, Glass NR, Sollinger H (1985) Neutropenic enterocolitis in adults: review of the literature and assessment of surgical intervention. Am J Surg 149:405–408
Slavin RE, Dias MA, Saral R (1978) Cytosine arabinoside induced gastrointestinal toxic alterations in sequential chemotherapeutic protocols: a clinical pathologic study of 33 patients. Cancer 42:1747–1759
Girmenia C, Micozzi A, Cartoni C, et al. (1999) Detection of Candida mannoproteinemia in patients with neutropenic enterocolitis. Eur J Clin Microbiol Infect Dis 18:55–58
Cartoni C, Dragoni F, Micozzi A, et al. (2001) Neutropenic enterocolitis in patients with acute leukemia: prognostic significance of bowel wall thickening detected by ultrasonography. J Clin Oncol 19:756–761
Von Lilienfeld-Toal M, Ebert O, Theuerkauf I, Glasmacher A, Schmidt-Wolf IGH (2001) Small bowel obstruction in acute myelogenous leukemia: stenosis or paralysis? Ann Hematol 80:611–613
Byrd JC, Edenfield J, Shields DJ, Dawson NA (1995) Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol 13:1800–1816
Corpechot C, Lemann M, Brocheriou I, et al. (1998) Granulocytic sarcoma of the jejunum: a rare cause of small bowel obstruction. Am J Gastroenterol 93:2586–2588
Schwyzer R, Sherman GG, Cohn RJ, Poole JE, Willem P (1998) Granulocytic sarcoma in children with acute myeloblastic leukemia and t(8;21). Med Pediatr Oncol 3:144–149
Kim H (1998) Primary granulocytic sarcoma of the duodenum: radiologic and endoscopic findings. Am J Roentgenol 170:1115–1116
Semelka RC, John G, Kelekis NL, Burdeny DA, Ascher SM (1996) Small bowel neoplastic disease: demonstration by MRI. J Magn Reson Imaging 6:855–860
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by MURST 40%
Rights and permissions
About this article
Cite this article
Capria, S., Vitolo, D., Cartoni, C. et al. Neutropenic enterocolitis in acute leukemia: diagnostic and therapeutic dilemma. Ann Hematol 83, 195–197 (2004). https://doi.org/10.1007/s00277-003-0755-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-003-0755-5