Abstract
Hepatic arterial infusion pumps are increasingly utilized as an option for liver directed therapy in the treatment of metastatic colorectal carcinoma. After skeletonization of the hepatic artery through the ligation of extra-hepatic branches, these pumps are implanted surgically with their tip placed in the common hepatic artery. Subsequently, a nuclear medicine pump study is performed to ensure homogeneous perfusion of the liver and detect any extrahepatic perfusion. We report a peripheral arc between the superior mesenteric artery and celiac axis, which caused misperfusion on the SPECT nuclear medicine scan.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is the most common site of metastatic disease from colorectal carcinoma [6, 9]. For patients with limited disease, surgical resection is shown to have durable long-term survival [3, 5, 12, 13]. Patients with unresectable disease have poor long-term prognoses [5, 12]. For these patients alternative strategies have been employed including hepatic arterial infusion chemotherapy [14]. This is accomplished by surgical implantation of infusion pumps placed with the catheter introduced into the gastroduodenal artery and the tip at its junction with the proper hepatic artery. Typically, 5-fluoro-2′-deoxyuridine (floxuridine, FUDR), is the chemotherapeutic agent of choice given its high intra-hepatic extraction [4]. Prior to initiation of chemotherapy, a radionuclide pump study is performed with macroaggregated albumin (MAA) tagged to technetium-99m injected through the infusion pump. Radioisotope distribution is imaged to assure homogeneous uptake throughout the liver. Nonuniform biodistribution is referred to as “misperfusion”. Misperfusion is often the result of aberrant anatomy not appreciated during pump placement.
We present the case of a 56-year-old gentleman who had altered pump biodistribution secondary to variant hepatic arterial anatomy and its subsequent treatment.
Case presentation
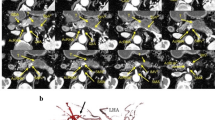
A 56-year-old male with a history of rectal adenocarcinoma developed disease recurrence in the liver following chemoradiation with the evidence of complete response. A hepatic arterial infusion pump was placed intraoperatively. A radionuclide pump study was performed to assess biodistribution 1 week after pump placement. SPECT CT demonstrated altered bio distribution of the radiopharmaceutical within the liver along with extrahepatic activity localizing to the head of the pancreas (Fig. 1). A CT angiogram was subsequently performed demonstrating proper position of the infusion catheter tip but aberrant hepatic arterial anatomy (Fig. 2). The patient underwent catheter angiography demonstrating an arc between the common hepatic artery and a replaced right hepatic artery arising from the superior mesenteric artery. It was felt that this variant arc was likely the cause of the abnormal hepatic perfusion on the radionuclide pump study. Arteriography of the common hepatic artery demonstrated an arc connecting the middle and left hepatic artery to a replaced right hepatic artery originating from the superior mesenteric artery (Fig. 3). The replaced right hepatic artery was then selected from the superior mesenteric artery and the catheter advanced to a position immediately proximal to the arc (Fig. 4). An Amplatzer plug (St. Jude Medical, Minneapolis, MN) was deployed immediately proximal to the aberrant arc (Fig. 5). Completion angiography through the hepatic artery demonstrated distal occlusion of the replaced right hepatic artery and no residual perfusion from the superior mesenteric artery (Fig. 6). Subsequent radionuclide pump study performed the same day demonstrated normal biodistribution of radiopharmaceutical (Fig. 7). The patient was discharged the same day and began receiving chemotherapy through his infusion pump.
CTA of the liver demonstrating the proper hepatic artery (double arrowhead),arc between distal proper hepatic artery and distal replaced right hepatic artery from superior mesenteric artery (arrow) and replaced hepatic artery from superior mesenteric artery single arrowhead. (C celiac axis, S superior mesenteric artery)
Discussion
Variant anatomy may go unrecognized intraoperatively and result in misperfusion on the immediate post-operative radionuclide scan. Misperfusion can be a source of increased morbidity for patients after pump insertion both from inadequate distribution of chemotherapy in the liver as well as result in extra-hepatic delivery of chemotherapeutic agents. It is important to identify misperfusion prior to the initiation of pump chemotherapy.
Variation in the anatomy of the celiac axis is common with “traditional” trifurcation (common hepatic, splenic, left gastric) present in 65% [7]. A review of variant anatomy in 600 patients by Covey described variant anatomy in 49% [2], while a review of CT and catheter angiograms in 5002 patients [11] described celiac and common hepatic artery variants in 11% of patients. It is important that these variations be appreciated before or at the time of surgical placement of hepatic artery pumps as they may lead to misperfusion. Common variations affecting pump placement are listed in Table 1.
The arc present in our patient was not described in the 200 dissections of Michaels [7] or 600 digital subtraction angiograms of Covey [2]. Song’s review of celiac and hepatic artery variations in 5002 patients describes ambiguous celiac anatomy with anastomotic connections between the celiac and superior mesenteric arteries [11]. While similar, the anastomosis in this case appears more peripheral than in illustration. A similar anastomosis is reported by Catalano but was between a replaced right hepatic artery from the SMA and accessory hepatic artery from the celiac axis [1].
The embryologic basis for the development of variant anatomy as attributed to Tandler is reviewed by Michaels [8]. During embryogenesis four ventral roots of the primitive aorta are destined to form the mesenteric vasculature. These roots are initially joined by anterior longitudinal anastomotic branches which selectively disappear as do proximal ventral roots #11 and 12 giving rise to the celiac axis and the superior mesenteric artery. Variations in persistence and disappearance of ventral roots and or longitudinal branches explain variant celiac and superior mesenteric anatomy and possibly the origin for this arch.
Arcs connecting branches of the mesenteric circulation have been previously described including the arcs of Beuhler, Barkow and Riolan as well as the pancreatico/duodenal arcade. The arc of Beuhler is a proximal anastomotic communication between the celiac axis and the superior mesenteric artery likely from failure of disappearance of longitudinal anastomosis between the 10tht and 13th ventral mesenteric roots. This arc would not influence pump placement. The arc described in this paper occurs at the level of the origins of the left hepatic and right hepatic arteries. If the arc was not present, this patient’s anatomy would be described as a left hepatic artery from the common and subsequently proper hepatic artery and a replaced right hepatic artery from the superior mesenteric artery. The location of this arc, however, permits both the SMA and celiac axis via the common hepatic artery to contribute perfusion to both the right and left lobes of the liver. This competitive liver perfusion explains the misperfusion seen on the radionuclide pump study. The extrahepatic perfusion was likely related to a small duodenal branch of the proximal gastroduodenal artery adjacent to the tip of the pump catheter which spontaneously occluded.
After the recognition of misperfusion, CT and catheter angiography can delineate causative variant anatomy. Transcatheter embolization using coils or plugs is commonly used to normalize liver perfusion and obviate the need for surgical intervention [10]. Choice of an Amplatzer plug in this case was in made to assure stable placement with less likelihood of the migration often seen with coils.
In summary, misperfusion needs to be recognized on post-operative nuclear scintigraphy prior to the initiation of pump chemotherapy, as it can result in significant patient morbidity. CT and catheter angiography are safe and effective ways of evaluating aberrant anatomy which may be the cause of the misperfusion. Arterial occlusion using plugs and coils have shown to be safe and effective means of restoring normal biodistribution and should be considered in patients with misperfusion.
References
Catalano OA et al (2008) Vascular and Biliary variants in the liver: implications for liver surgery Radiographics 28(2):359–378
Covey AM et al (2002) Variant Hepatic arterial anatomy revisited: Digital subtraction angiography performed in 600 patients. Radiology 224(2):542–547
Creasy JM et al (2018) Actual 10-year survival after hepatic resection of colorectal liver metastases: what factors preclude cure? Surgery 163(6):1238–1244
D’Angelica MI et al (2015) Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg 261(2):353–360
De Jong MC et al (2009) Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patient. Ann Surg 250(3):440–448
Hess KR et al (2006) Metastatic patterns in adenocarcinoma. Cancer 1;106(7):1624–1633
Michels NA. (1966) Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg. 112(3):337–347
Michels NA (1955) Blood supply and anatomy of the upper abdominal organs. J.B.Lippincott, Philadelphia
Rihimaki M et al (2016) Patterns of metastasis in colon and rectal cancer. Sci Rep 15:6:29765
Sofocleous CT et al (2006) Arterial embolization for salvage of hepatic artery infusion pumps. J Vasc Interv Radiol 17(5):801–806
Song SY et al (2010) Celiac axis and common hepatic artery variations in 5002 patients: systematic analysis with spiral CT and DSA. Radiology 255(1):278–288
Tomlinson JS et al (2007) Actual 10 year survival after resection of colorectal liver metastasis defines cure. J Clin Oncol 25(29):4575–4580
Vigano L et al (2008) Liver surgery for colorectal metastases: results after 10 years follow up. Long term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol 15(9):2458–2464
Zervoudakis A et al (2017) Treatment options in colorectal liver metastases: hepatic arterial infusion. Visc Med 33(1):47–53
Author information
Authors and Affiliations
Contributions
OH: Manuscript writer/editor, interventional radiology fellow, participated in the interventional radiology portion of the case. SG: Manuscript writer/editor, surgical oncology fellow, participated in the surgical portion of the case. TK: Manuscript writer/editor, attending surgeon for the pump insertion. DC: Manuscript writer/editor, attending surgeon assisting with pump insertion. JK: Manuscript writer/editor, nuclear medicine attending, interpreted pump studies. JN: Manuscript writer/editor, interventional radiology attending for angiographic portion of the case.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Hasan, O., Greco, S., Kennedy, T. et al. Aberrant arc between the common hepatic artery and a replaced right hepatic artery resulting in misperfusion in a patient with a hepatic arterial infusion pump. Surg Radiol Anat 41, 355–358 (2019). https://doi.org/10.1007/s00276-018-2158-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-018-2158-2