Abstract
Background
Changes in head position are thought to cause a degree of brain shift in the intracranial cavity. However, little is known on the concurrent shift of the cerebral veins. The present study aimed to investigate the positional shift of the cerebral veins that accompanies brain shift.
Methods
Sagittal T2-weighted magnetic resonance imaging was performed on 21 consecutive patients lying in the supine and prone positions, using the same sequence. For each patient, imaging data were obtained for the two positions as a pair of images with morphologically best-matched cerebral contours.
Results
The subarachnoid spaces in the parasagittal frontal convexity showed variable reductions related to a postural change from a supine to a prone position, with a mean percent reduction (%Δ) of 17.8 ± 11.7%. Additionally, cerebral cisterns ventral to the brainstem and upper cervical cord were reduced in most patients when lying in a prone position, with a mean %Δ of 16.6 ± 8.7%. In contrast, none of these 130 pairs of identical venous segments located in the parasagittal cerebral convexity showed positional shift. Cadaveric dissections found that the major cortical veins were superficially upheld by the arachnoid membranes.
Conclusions
The parasagittal major cortical and bridging veins do not seem to show positional shifts. Positional change in the posterior–anterior direction causes a shearing between the frontal cortices and the distributing veins and can be a risk factor for acute subdural hemorrhage, in case of severe head trauma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In general, traumatic acute subdural hemorrhage is acknowledged as a serious condition and typically results in a poor outcome [5]. Since traumatic injury of the intracranial bridging veins connecting the cerebral dural sinuses and the cortical veins can result in a lethal acute subdural hemorrhage, the bridging veins have been a subject of interest and extensively explored [2, 3, 8, 9, 12]. Additionally, intracranial brain shift and deformation caused during head trauma have been quantitatively examined by measuring the relative brain-skull displacement in experimental models [1, 4]. In contrast, intraoperative brain shift that depends on the patient’s head position and gravity and that is critical for accurate surgeries, has been evaluated in the context of craniotomies [6]. Furthermore, positional brain shift in different physiological conditions has been studied in healthy volunteers [7, 10, 11]. However, to our knowledge, no study has documented positional shift of the cortical and bridging veins. Nevertheless a recent investigation suggested that some bridging veins located in the cerebral convexity are suspended by hanging-type arachnoid sleeves continuous with the arachnoid granulations that form a pile that drives into the skull [14].
The present study aimed to investigate the positional shift of the parasagittal cortical and bridging veins that occurs at the same time as a shift of the brain.
Materials and methods
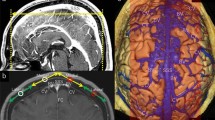
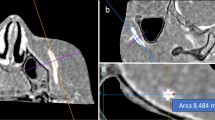
This retrospective study included 21 patients who visited our hospital on an outpatient basis between March and August 2011. These patients presented with headaches, dizziness, tinnitus, hearing disturbances, hemisensory disturbances, or seizure. The patients comprised 15 men and 6 women, with a mean age of 46.8 years (range 32–79 years). Initial examinations using axial T1-weighted and T2-weighted images, T2-gradient echo, fluid-attenuated inversion recovery, and diffusion-weighted sequences confirmed that none of these patients had a brain tumor, cerebral infarct, intracerebral hemorrhage, or hydrocephalus. The patients then underwent a thin-sliced, sagittal T2-weighted sequence both in supine and prone positions, consecutively; both scans were performed with the patient’s neck adjusted in the same curvature against the examining table. The head was scanned for 40 mm between both parasagittal regions with the superior sagittal sinus centered, providing 20 slices in each position. The following parameters were adopted in both positions: repetition time (TR), 3500.00 ms; echo time (TE), 90.00 ms; slice thickness, 2.00 mm; interslice gap, 0 mm; matrix, 300 × 189; field of view, 200 mm × 200 mm × 40 mm; flip angle, 90°; and scan duration, 2 min 40 s. All imaging sequences were obtained using a 3.0 T MR scanner (Achieva R2.6; Philips Medical Systems, Best, The Netherlands). Imaging data were transferred to a workstation (Virtual Place Lexus64. 64 edition; AZE, Tokyo, Japan), individually saved in two files according to the scanned posture, and independently analyzed by two of the authors (S.T. and Y.Y.). From each file containing 20 images, two with morphologically best-matched cerebral contours were selected as a pair, targeted for two different cerebrospinal fluid (CSF)-filled regions. One pair was selected from the parasagittal region for evaluating the frontal subarachnoid spaces, and the other pair was from the midsagittal region for evaluating the cerebral cisterns ventral to the brainstem. From these pairs of images, areas of the subarachnoid spaces in the parasagittal frontal convexity and midsagittal cisterns ventral to the brainstem and upper cervical cord were calculated on the workstation by manually tracing the outer margin of these CSF-filled regions. In each patient, the measurement procedure was performed three times for each CSF-filled region and the mean value was recorded as data. The upper limit of the frontal subarachnoid spaces was defined as the lower margin of the coronal suture and the lower limit of it was defined as the floor of the anterior fossa (Fig. 1a, b). The upper limit of the cerebral cisterns ventral to the brainstem was defined as the line connecting the upper margins of the pons and dorsum sellae. The lower limit of the cisterns was defined as the level of the upper cervical cord where the ventral dura mater changes direction at the craniocervical junction (Fig. 1c, d). Furthermore, the sites and number of identical venous segments located at the parasagittal cerebral convexity were recorded on the pair of images.
Parasagittal (a, b) and midsagittal (c, d) T2-weighted magnetic resonance images of the same patients show a reduction in the area (Δ) of the frontal subarachnoid spaces (a, b colored in red) and cerebral cisterns ventral to the brainstem and upper cervical cord (c, d colored in red), in response to positional changes. These are calculated by As − Ap. CS coronal suture, DS dorsum sellae, As area in supine position, Ap area in prone position; dotted line in a, b lower margin of the CS; dotted line in c, d a line connecting between the upper margins of the pons and dorsum sellae. (Color figure online)
Dissection on both sides of three injected postmortem heads was carried out at the Department of Neurological Surgery, University of Florida, Gainesville, FL, USA.
The present study was performed in accordance with the guidelines of our institution regarding human research. Written informed consent was obtained from all patients prior to their participation in this study.
Results
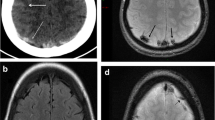
In all, the subarachnoid spaces in the parasagittal frontal convexity showed variable degrees of reduction related to postural change from a supine to prone position (Fig. 2a, b). The mean area of these subarachnoid spaces was measured as 405.9 ± 150.9 mm2 (range 129–673 mm2) in the supine and 341.7 ± 147.4 mm2 (range 73–580 mm2) in the prone position, with a mean percent reduction (%Δ) of 17.8 ± 11.7% (range 2–43%) (Fig. 1a, b). Additionally, cerebral cisterns ventral to the brainstem and upper cervical cord were reduced to variable degrees in 20 of the 21 patients lying in a prone position (Fig. 2c, d). The mean area of these cisterns were measured as 388.1 ± 88.7 mm2 (range 224–501 mm2) in the supine and 322.5 ± 81.3 mm2 (range 199–502 mm2) in the prone position, with a mean %Δ of 16.6 ± 8.7% (range 2–43%) (Fig. 1c, d). In one patient, the cisterns increased by 2% when moving into the prone from the supine position (Table 1). These %Δ demonstrated that the area of the subarachnoid spaces was greater at the supine position. Furthermore, a total of 130 pairs of identical venous segments were identified in the 21 patients in the parasagittal cerebral convexity, with a mean of 6.2 (range 4–9). None of these showed an identifiable positional shift (Fig. 3).
Parasagittal (a, b) and midsagittal (c, d) T2-weighted magnetic resonance images of the same patients show extensions of the subarachnoid spaces at the frontal tip (a, b arrowheads) and cerebral cisterns ventral to the brainstem and upper cervical cord (c, d arrowheads), in both supine and prone positions. Arrows cortical and bridging veins lying adjacent to the dura mater
The frontotemporal craniotomies performed in the postmortem heads consistently showed that the major cortical veins course superficially, floating from the cerebral cortices and upheld by the arachnoid membranes (Fig. 4).
A left frontotemporal craniotomy performed in a postmortem specimen shows the cerebral cortices after reflection of the dura mater (a) and after removal of the arachnoid membranes (b). Note that a major cortical vein (a, b arrowheads) courses superficially, floating from the cerebral cortices and is upheld by the arachnoids (a arrowheads). AM arachnoid membrane, FB frontal bone, IFG inferior frontal gyrus, TB temporal bone, TL temporal lobe, TM temporalis muscle
Discussion
In the present study, the subarachnoid spaces in the parasagittal frontal convexity and the cisterns ventral to the brainstem showed variable degrees of reduction by postural change from the supine to prone position. Furthermore, the degree of the brain shift demonstrated a positive relationship with the area of the parasagittal frontal subarachnoid spaces at the supine position, suggesting that patients with considerable atrophy in the frontal cortices can sustain a larger positional brain shift.
In contrast, positional shift was not identified in the venous segments located at the parasagittal convexity. Furthermore, cadaveric dissections consistently showed that the major cortical veins coursed superficially, upheld by the arachnoid membranes. These findings may support the notion that the parasagittal major cortical and bridging veins do not sustain positional shift in the posterior–anterior direction. This also means that positional change can cause shearing between the cerebral cortices and distributing veins.
Therefore, we concluded that a positional change in the posterior–anterior direction can be a risk factor for acute subdural hemorrhage, especially in head impacts accompanying decelerating and accelerating forces. This conclusion is consistent with that in a previous report showing that falls with accompanying positional changes were associated with a larger degree of brain–skull displacement and a higher risk to bridging vein ruptures compared to punches without accompanying positional changes [1].
Also, the outcomes obtained in the present investigation have clinical relevance with the underlying mechanism of the “Shaken Baby” syndrome that is represented by the triad of subdural hemorrhage, retinal hemorrhage, and encephalopathy [13]. Yilmaz et al. documented an interesting neuroimaging finding that multifocal signal loss at bridging veins was present on susceptibility-weighted imaging in infants with abusive head trauma [15].
There are several limitations of the present study. In contrast with previous investigations using three-dimensional model registration [6, 7, 10, 11], the methodology of the present study was simpler and easier to perform. It was based on two-dimensional analysis where the targeted CSF-filled areas were measured on a pair of MR images both at supine and prone positions, followed by a comparison of the outcomes in both positions. The positional shift was examined only in the posterior–anterior direction and was not explored in the lateral direction. Sites for exploration were limited to two regions of the brain. In addition, the patient population was small and heterogeneous for age. Thus, obtained results were not statistically analyzed. In spite of these defaults and limitations, we believe that the present study could indicate the possibility of shearing between the cerebral cortices and perfusing veins caused in response to a positional change. However, further investigation in a much larger population is needed to verify the outcomes of the present study.
Conclusions
The parasagittal major cortical and bridging veins seem not to sustain positional shifts. Positional change in the posterior–anterior direction causes a degree of shearing between the frontal cortices and distributing veins that could be a risk factor for acute subdural hemorrhage, in case of severe head trauma.
References
Baumgartner L, Schmitt KU (2014) Computer simulations to investigate the consequence of blunt head impact. J Forensic 59:1191–1197
Brockmann C, Kunze SC, Schmiedek P, Groden C, Scharf J (2012) Variations of the superior sagittal sinus and bridging veins in human dissections and computed tomography venography. Clin Imaging 36:85–89
Delye H, Goffin J, Verschueren P, Vander Sloten J, Van der Perre G, Alaerts H, Verpoest I, Berckmans D (2006) Biomechanical properties of the superior sagittal sinus-bridging vein complex. Stapp Car Crash J 50:625–636
Feng Y, Abney TM, Okamoto RJ, Pless RB, Genin GM, Bayly PV (2010) Relative brain displacement and deformation during constrained mild frontal head impact. J R Soc Interface 7:1677–1688
Hamed M, Schuss P, Daher FH, Borger V, Güresir Á, Vatter H, Güresir E (2016) Acute traumatic subdural hematoma: surgical management in the presence of cerebral herniation—a single-center series and multivariate analysis. World Neurosurg 94:501–506
Hill DL, Maurer CR Jr, Maciunas RJ, Barwise JA, Fitzpatrick JM, Wang MY (1998) Measurement of intraoperative brain surface deformation under a craniotomy. Neurosurgery 43:514–528
Monea AG, Verpoest I, Vander Sloten J, Van der Perre G, Goffin J, Depreitere B (2012) Assessment of relative brain-skull motion in quasistatic circumstances by magnetic resonance imaging. J Neurotrauma 29:2305–2317
Morandi X, Haegelen C, Henaux PL, Riffaud L (2006) Brain shift is central to the pathogenesis of intracerebral haemorrhage remote from the site of the initial neurosurgical procedure. Med Hypotheses 67:856–859
Mortazavi MM, Denning M, Yalcin B, Shoja MM, Loukas M, Tubbs RS (2013) The intracranial bridging veins: a comprehensive review of their history, anatomy, histology, pathology, and neurosurgical implications. Childs Nerv Syst 29:1073–1078
Roberts DR, Zhu X, Tabesh A, Duffy EW, Ramsey DA, Brown TR (2015) Structural brain changes following long-term 6° head-down tilt bed rest as an analog for spaceflight. AJNR Am J Neuroradiol 36:2048–2054
Schnaudigel S, Preul C, Ugur T, Mentzel HJ, Witte OW, Tittgemeyer M, Hagemann G (2010) Positional brain deformation visualized with magnetic resonance morphometry. Neurosurgery 66:376–384
Schuenke M, Schulte E, Schumacher U, Ross L (2007) Head and neuroanatomy. Thieme atlas of anatomy. Thieme, Stuttgard, p 256
Squire W (2011) The “Shaken Baby” syndrome: pathology and mechanisms. Acta Neuropathol 122:519–542
Tsutsumi S, Ono H, Yasumoto Y (2016) Pile driving into the skull and suspending the bridging veins? An undescribed role of arachnoid granulations. Surg Radiol Anat (Epub ahead of print)
Yilmaz U, Körner H, Meyer S, Reith W (2015) Multifocal signal loss at bridging veins on susceptibility-weighted imaging in abusive head trauma. Clin Neuroradiol 25:181–185
Acknowledgements
This study did not receive any grant funding.
Author contributions
All authors contributed equally to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare regarding the materials or methods in this study or the findings specified in this paper.
Rights and permissions
About this article
Cite this article
Tsutsumi, S., Ono, H. & Yasumoto, Y. Immobile cerebral veins in the context of positional brain shift: an undescribed risk factor for acute subdural hemorrhage. Surg Radiol Anat 39, 1063–1067 (2017). https://doi.org/10.1007/s00276-017-1837-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-017-1837-8