Abstract
Purpose
The aim of this study is to investigate the detailed anatomy of the posterior talofibular ligament (PTFL) on MR images in patients with os trigonum. We also evaluated the pathological conditions of the PTFL, anterior talofibular ligament (ATFL), flexor hallucis longus (FHL) tendon, talus and os trigonum.
Methods
Ankle MRIs of 70 patients with os trigonum (study group) and 70 patients without it (control group) were reviewed for the anatomy of the anterior and posterior fibers of PTFL. The prevalence of PTFL and ATFL pathologies was also compared between two groups. Additionally FHL tenosynovitis and osseous pathologies were evaluated.
Results
The posterior fibers inserted into the lateral tubercule of the posterior process of the talus in the control group whereas if an os trigonum was present, the posterior fibers of PTFL were inserted only into the os trigonum. The origins of anterior and posterior fibers were the medial surface of the lateral malleolus and the insertion of the anterior fibers was lateral surface of the talus posterior to the lateral malleolar facet in both groups. There was a significant association between an abnormal PTFL, ATFL and the presence of os trigonum. FHL tenosynovitis was higher in the study group but it did not meet the statistical significance. The most common pathology of the talus and os trigonum was subchondral edema along the synchondrosis.
Conclusions
In patients with os trigonum, the posterior fibers of the PTFL were inserted herein. In the case of an os trigonum signal alterations of ligaments were more common, which may reflect chronic instability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Os trigonum is an accessory ossicle resulting from the failure of a secondary ossification center and located posterior to the talus. It is present in ~7 % of adults [1]. It usually forms between 7 and 13 years of age and fuses to the talus within 1 year of its appearance in the majority of patients, or persists as os trigonum [5, 11]. Os trigonum is a triangular accessory ossicle fusing to the lateral tubercle of the posterior process of the talus. Os trigonum syndrome has been named for the group of symptoms resulting from trigonal process or os trigonum pathologies. This syndrome is one of the most common causes of posterior heel pain causing posterior ankle impingement [12].
Presence of an os trigonum can often be identified on plain radiographs, but magnetic resonance imaging (MRI) is the modality of choice for the evaluation of an os trigonum and concurrent ligamentous, tendinous and osseous pathologies. Even if two different bundles of the ligament are described as anterior and posterior fibers in the anatomical literature, the detailed anatomy of the posterior talofibular ligament (PTFL) has not been discussed in the radiological literature. No study exists in the literature regarding the evaluation of the anatomy and variations of the PTFL if an os trigonum presents. Presence of os trigonum is not only an anatomical variation but also may change the mechanics of the ankle and may be considered as a risk factor for instability of the lateral ankle. This condition may lead to injury of the ligaments. PTFL abnormalities associated with os trigonum have not been discussed in the literature as well.
We aim to investigate the detailed anatomy of the PTFL on MR images and showed the origin and insertion of the anterior and posterior fibers in subjects with os trigonum. We also evaluated the concomitant pathological conditions of the PTFL, anterior talofibular ligament (ATFL), tendon of the flexor hallucis longus (FHL) muscle, talus and os trigonum.
Materials and methods
This retrospective study was approved by the Institutional Review Board of our hospital. Due to the retrospective nature of the study, informed consent by patients and providers was not required.
Patient inclusion
Seventy consecutive patients presenting with os trigonum (study group) and seventy patients without os trigonum (control group) evaluated by MRI in the past 5 years were identified via a radiology information system keyword search. The ankle MRIs and medical data, including demographic information (age and gender) and clinical history of all patients were analyzed. The study group consisted of 70 patients with os trigonum, 28 females (40 %) and 42 males (60 %) with an average age of 38.6 years (range: 12–75 years, standard deviation: 15.94). The control group consisted of 70 patients without os trigonum, 42 females (60 %) and 28 males (40 %) with an average age of 41.6 years (range: 17–69 years, standard deviation: 14.87; Table 1). We excluded patients with a history of acute trauma (the injury was considered acute if less than 4 weeks had elapsed between the time of trauma and the MRI examination), MRIs of poor quality, incomplete demographic or clinical information, or MRI features suggestive of acute trauma although no history of trauma was reported. The patients with a history of previous ankle or foot surgery were also excluded from the study. The selection for the control group was made using the same exclusion criteria as for the study group in the absence of os trigonum. The patients without any exclusion criteria were included into the study.
MRI review
The clinical indications for ankle MRI in the study and the control groups are listed in Table 2, respectively.
The scans were obtained on a 1.5-Tesla Signa MR unit (General Electric Medical Systems, Milwaukee, WI, USA) in conjunction with the extremity coil with the patient in a supine position. Standard sequences were obtained for all ankle MRI scans, which included sagittal, axial and coronal proton density (PD) fat-suppressed images, axial fast-spin-echo T2-weighted fat-suppressed images and axial and coronal fast-spin-echo T1-weighted images. T1-weighted images were obtained with 500/20 (TR/TE), a 320 × 288 acquisition matrix, a 16-cm field of view, and two excitations. PD fat-suppressed images were obtained with 2840/42 (TR/TE), a 320 × 256 acquisition matrix, a 16-cm field of view, and two excitations. T2-weighted images were obtained with 3550/60 (TR/TE), a 320 × 256 acquisition matrix, a 16-cm field of view, and two excitations. For all patients, the slice thickness was 3 mm and the interslice gap was 1 mm. MR images were also obtained digitally using a picture archiving communication system (PACS), and MRI evaluation was performed using the PACS software. Ankle MRIs were blindly reviewed by an attending musculoskeletal radiologist with 10 years of experience in the characteristics of the os trigonum, specifically for the origin and insertion of PTFL anterior and posterior fibers on axial MR images. Concurrent PTFL pathologies were also reviewed and categorized as normal or pathological (increased intraligamentous signal intensity representing edema, partial tear or complete tear). PTFL often appears striated on MRI secondary to its fibrofatty composition, which was accepted as normal in our study. PTFL was considered pathological when thickened and with heterogeneous high signal intensity on MRI. Partial or complete discontinuity in the ligament surrounded by fluid was accepted as a partial or complete tear.
Pathologies of the ATFL, FHL tendon and osseous structures (talus, os trigonum) were also reviewed and categorized as normal or pathological. Pathological ATFL conditions were thickening of the ligament and increased intraligamentous signal intensity representing edema, thickening of the ligament and decreased signal intensity representing fibrosis, partial, or complete tearing of the ligament.
The relationship between os trigonum and FHL tendon has been widely discussed in the literature previously. In this study we evaluated the concomitant pathological conditions of the FHL tendon and discussed with the literature. The abrupt cutoff of the FHL tendon synovial sheath fluid at the posterior talus and abnormal FHL tendon signal were considered pathological.
Subchondral edema, subchondral cysts along the synchondrosis and osteophytes were also accepted as pathological for the os trigonum and talus in the study group.
We also retrospectively evaluated ankle MRIs of 70 normal subjects without os trigonum and reviewed the MR examinations in terms of the PTFL anatomy and the abnormalities of the PTFL, ATFL and FHL.
Statistical analyses
Data analyses were performed using SPSS, version 15.0. Independent sample t test was used to compare the age distribution between the groups. The frequency distributions of PTFL pathologies in the two groups (the study and the control group) were examined using X 2 analysis. The alpha level of statistical significance was set at 0.05.
Results
There was no significant difference in the age distribution of the two groups (p = 0.254). In the study group, a significant difference was found in the distribution of os trigonum between male and female patients, with a significantly higher prevalence in males (p = 0.018).
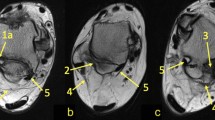
The PTFL was optimally visualized in the axial plane. The PTFL has a trapezoid shape and consists of two fibers; short anterior transverse and long posterior (Fig. 1). The origin of both fibers was the medial surface of the lateral malleolus in the control and study groups. The insertion of the anterior fibers was identical in both groups; they were attached to the lateral surface of the talus posterior to the lateral malleolar facet. The posterior fibers were directed posteromedially and inserted into the lateral tubercule of the posterior process of the talus in the control group (Fig. 2a). When os trigonum was present (study group), the posterior fibers were inserted only into the os trigonum (Fig. 2b).
a 42-year-old female with chronic ankle pain without os trigonum. Axial T2-weighted fat-suppressed image of the ankle at the level of talofibular ligaments shows the insertion of the posterior fibers of the posterior talofibular ligament (PTFL; white arrow) into the lateral tubercule of the posterior process of the talus (arrowhead). Dotted arrow indicates the anterior fibers of the PTFL. b 35-year-old asymptomatic female with os trigonum. Axial T2-weighted fat-suppressed image of the ankle at the level of talofibular ligaments shows the insertion of the posterior fibers of the posterior talofibular ligament (PTFL; white arrow) into the os trigonum (asterisk). Dotted arrow indicates the anterior fibers of the PTFL
The prevalence of the PTFL abnormality differed significantly between the groups, and PTFL pathologies were more common in the study group (p = 0.001) (Table 3). Twenty-seven patients in the study group had an abnormal PTFL on MR images, all had high signal intensity (edema) and none had partial or complete tearing of the ligament (Fig. 3). According to the information obtained from the hospital data system, all of these patients were symptomatic and had ankle pain. In the control group, only four patients had a concurrent PTFL pathology showing increased intraligamentous signal intensity. This increased signal intensity was located in both anterior and posterior fibers of the PTFL in the study and control groups. No patient in the control group had partial or complete tearing of the PTFL (Table 3).
The ATFL abnormality was significantly higher in the study group (p = 0.026) (Fig. 4). The most common pathology was the thickening of the ligament and decreased signal intensity representing fibrosis in both groups.
38-year-old male with chronic ankle pain. a Axial T2-weighted fat-suppressed image. b Axial FSE T1-weighted image shows complete disruption of the anterior talofibular ligament (white and black solid arrows). Open arrow indicates os trigonum with subchondral edema. Note the edema in the talus along the synchondrosis
The prevalence of FHL tendon pathologies was higher in the study group, but the difference was not significant (p = 0.217) (Fig. 5). Ten patients in the study group and 1 patient in the control group had simultaneously both PTFL and FHL tendon pathologies on MR images.
50-year-old female with chronic ankle pain. a Axial T2-weighted fat-suppressed image shows loculated fluid in flexor hallucis longus tendon sheath (arrow). b Sagittal proton density fat-suppressed image of the ankle shows os trigonum with abrupt cutoff of fluid at the level of the posterior talus (dotted arrow). Flexor hallucis tendon was normal (not shown). Open arrow indicates os trigonum
The most common pathology of the talus (40 %) and the os trigonum (47.1 %) along the synchondrosis was subchondral edema. The patients who have subchondral cysts were 21.4 % and osteophytes were 21.4 % in the talus. The patients who have subchondral cysts were 27.1 % and osteophytes were 24.2 % in os trigonum. Eleven patients (17.2 %) had more than one abnormalities in the talus, and eighteen patients (1.6 %) in the os trigonum (Fig. 6).
Discussion
PTFL is visualized between talus and the medial surface of the lateral malleolus in a trapezoid shape on the axial MR images. Even if two different bundles of the ligament are described as anterior and posterior fibers in the anatomical literature, the detailed anatomy of the PTFL has not been discussed in the radiological literature. The origins of the anterior and posterior fibers of the PTFL are the medial surface of the lateral malleolus of the fibula. The anterior fibers of the PTFL were inserted into the lateral surface of the talus posterior to the lateral malleolar facet. The posterior fibers were inserted into the lateral tubercle of the posterior process of the talus. In this study we aimed to investigate the anatomy of the PTFL on MR images and showed the origin and insertion of the anterior and posterior fibers in patients with os trigonum. We also evaluated the potential pathologies of the PTFL in these patients. In our knowledge the pathological conditions of the PTFL if an os trigonum presents have not been reported in the literature. The abnormalities of the ATFL, FHL and osseous structures were also described.
There are only a few studies about the anatomy of the PTFL and the insertion of the ligament was described as os trigonum if it presents in the literature [3]. However, the anterior and posterior fibers of the PTFL were not evaluated separately in the literature in the cases with os trigonum. In this study the origin of the anterior and posterior fibers and the insertion of the anterior fibers were identical in the control and study groups. The anterior fibers were inserted into the lateral surface of the talus posterior to the lateral malleolar facet. If an os trigonum was present, the posterior fibers of the PTFL attached to the os trigonum. In our knowledge this is the first detailed description of the PTFL anatomy in terms of bundles if os trigonum presents. The posterior fibers were inserted into the lateral tubercule of the posterior process of the talus in the control group as reported in the literature. Courvoisier et al. [2] demonstrated that the posterior fibers of the PTFL in human cadavers attached to the medial tubercle of the posterior process of the talus, likely because the fibers would have to cross the tendon of the flexor hallucis longus either on its back or anterior aspect, which does not occur. Thus, the main attachment of the PTFL posterior fibers is to the lateral tubercle of the posterior process of the talus. As this lateral tubercle may be replaced in ~7–10 % of humans (more frequently in males) by an os trigonum, these fibers are then attached to this isolated ossicle. Furthermore, Courvoisier et al. [2] indicated that anterior fibers of the PTFL attached to the posterior edge of the talus, which would be possible only if the anterior fibers were longer than the posterior and crossed the posterior fibers, which is unlikely. Anatomically, the anterior fibers are shorter than the posterior bundle and attach to the lateral surface of the talus posterior to the lateral malleolar facet; i.e., the articular facet on the lateral aspect of the talus serves as the counterpart to the malleolus lateralis on the fibula.
Golano et al. [3] investigated the anatomy of the ankle ligaments; however, the PTFL was not divided into anterior and posterior fibers. They demonstrated that the posterior talofibular ligament was originated from the malleolar fossa which was located on the medial surface of the lateral malleolus. The insertion location of the PTFL was not in a specific area due to its multifascicular nature, and was inserted most frequently into the posterior surface of the talus or os trigonum, if present.
When an os trigonum is present, this accessory ossicle along with surrounding soft tissues can become wedged between the tibia, talus and calcaneus due to repetitive ankle plantar flexion, which can lead to inflammation of the involved structures. This pathological condition is referred to as os trigonum syndrome, talar compression syndrome or posterior tibiotalar impingement syndrome [10]. Knowledge of the morphology and anatomy of this accessory ossicle is important for understanding the possibility of injury to this area. The os trigonum syndrome and associated osseous and tendinous pathologies, such as FHL tenosynovitis, are well described in the literature. However, concurrent ligamentous injury has not been discussed in detail. In this study, the prevalence of PTFL and ATFL pathologies were also compared between patients with and without os trigonum. We demonstrated a statistically significant association between an abnormal PTFL, ATFL and the presence of os trigonum. Our findings corroborate the notion that the developmental nature of os trigonum may be a risk factor for impaired stability of the ankle. Indirect stress on the ATFL and PTFL, particularly on the posterior fibers, which insert into the os trigonum, may contribute to the instability of the ankle. The PTFL can be damaged due to a severe inversion stress; such an injury usually accompanies injury to the ATFL and calcaneofibular ligaments, components of the lateral collateral ligament complex of the ankle [10]. However, several patients in our study group had an abnormal PTFL without any ATFL injury. We suggest that the PTFL may become susceptible to injury due to anatomic variations, such as os trigonum. Lee et al. [8] suggested that PTFL injury was a significant factor affecting the anterior translation of the talus, as observed on stress radiographs. They also stated that other factors, such as age, gender, and personal characteristics should be considered when defining lateral ankle instability. In our study, the os trigonum, an accessory ossicle of the ankle, was considered a personal anatomical variation that contributes to the instability of the ankle. This retrospective study, which aims to define the radiopathologic findings associated with os trigonum, can be regarded as a preliminary study. A prospective study including detailed physical examination and MRI findings may provide new and comprehensive information.
Coexistence of os trigonum and tenosynovitis of the FHL tendon has been described in numerous studies in the literature [6, 9, 13]. Os trigonum can compress the FHL tendon when the tendon courses between the medial and lateral tubercule of the talus in the region of the fibro-osseous tunnel and causes the entrapment of the tendon [14]. Isolated fluid in the synovial sheath may indicate tenosynovitis. Since communication occurs between the FHL tendon synovial sheath and the ankle joint, the radiologist should be careful not to diagnose physiological synovial fluid as pathological [9, 15]. Lo et al. [9] considered the large amount of fluid surrounding the FHL tendon with abrupt cutoff of fluid at the posterior of the talus as abnormal; the same finding suggestive of entrapment was used in our study. Alterations in the signal intensity of the tendon were also accepted as pathological. A prevalence of the FHL tendon pathologies was higher in the study group although without statistical significance (p = 0.217).
The prevalence of osseous pathologies along the synchondrosis was evaluated in our study. The most common pathology of the talus and os trigonum was subchondral edema along the synchondrosis. Subchondral cysts and osteophytes were detected as an only pathologic condition in the bones in some of the patients or in conjuction with the other bone abnormalities in the others. We think that these pathologic conditions may be sequelae due to former trauma or instability of the ankle.
Os trigonum syndrome can be described accurately with the MRI. MRI is also the imaging method of choice to demonstrate the lateral collateral ligament complex. CT has mostly been used to search for subtle fractures in complicated ankle sprains, but the literature is very restricted about its use for imaging of the associated ligament injury. In this few reports the ATFL could be clearly visualized in all patients and the PTFL and calcaneofibular ligament could be depicted completely in a large portion of patients [4, 7].
Due to the retrospective nature of the study, patients with a history of asymptomatic chronic injury could not be excluded, which was the main limitation of our study. Since this limitation was valid for both groups, our statistical results may not have been affected.
The weak points of this study are that it is retrospective and it is not a cadaveric study. Further studies which are going to investigate the anatomical structures and pathological conditions related with os trigonum on cadaveric specimens to obtain MR anatomical correlation may be carried out to provide comprehensive information about the anatomy of PTFL if an os trigonum presents.
In conclusion, we demonstrated detailed anatomy of the PTFL in terms of bundles in cases with os trigonum. In our knowledge this is the first radioanatomical study which evaluates the PTFL if os trigonum presents on MR images. Familiarity with the radiological appearance of the anatomical variations is important for accurate evaluation of the pathologies. We also found a statistically significant association between the abnormalities of PTFL, ATFL and os trigonum. These findings, which may lead to instability of the ankle, may be investigated with a prospective clinical study. A prospective study including detailed physical examination and MRI findings may provide new and comprehensive information in this regard. Our findings may prompt further biomechanical studies of the pattern of potential ankle instability in patients with os trigonum.
References
Cerezal L, Abascal F, Canga A et al (2003) MR imaging of ankle impingement syndromes. AJR 181:551–559
Courvoisier A, Vialle R, Thevenin-Lemoine C, Mary P, Damsin JP (2008) The posterior talofibular ligament: an anatomical study with clinical implication in clubfoot surgery. Surg Radiol Anat 30:633–637
Golano P, Vega J, De Leeuw PAJ et al (2010) Anatomy of the ankle ligaments: a pictorial essay. Knee Surg Sports Traumatol Arthrosc 18:557–569
Gossner J (2010) Visibility of the lateral collateral ligaments in routine computed tomography of the ankle. Surg Radiol Anat 4:417–418
Grogan DP, Walling AK, Ogden JA (1990) Anatomy of the os trigonum. J Pediatr Orthop 10:618–622
Hamilton WG (1982) Stenosing tenosynovitis of the flexor hallucis longus tendon and posterior impingement upon the os trigonum in ballet dancers. Foot Ankle 3:74–80
Hua J, Xu JR, Gu HY et al (2008) Comparative study of the anatomy, CT and MR images of the lateral collateral ligaments of the ankle joint. Surg Radiol Anat 30:361–367
Lee KM, Chung CY, Kwon SS et al (2013) Relationship between stress ankle radiographs and injured ligaments on MRI. Skeletal Radiol 42:1537–1542
Lo LD, Schweitzer MR, Fan JK et al (2001) MR imaging findings of entrapment of the flexor hallucis longus tendon. AJR Am J Roentgenol 176:1145–1148
Maquirriain J (2005) Posterior ankle impingement syndrome. J Am Acad Orthop Surg 13:365–371
McDougall A (1955) The os trigonum. J Bone Joint Surg 37:257–265
Peace KAL, Hilier JC, Hulme A, Healy JC (2004) MRI Features of posterior ankle impingement syndrome in ballet dancers: a review of 25 cases. Clin Radiol 59:1025–1033
Sammarco GJ, Cooper PS (1998) Flexor hallucis longus tendon injury in dancers and nondancers. Foot Ankle 19:356–362
Sanhudo JA (2002) Stenosing tenosynovitis of the flexor hallucis longus tendon at the sesamoid area. Foot Ankle Int 23:801–803
Schweitzer ME, Van Leersum M, Ehrlich SS et al (1994) Fluid in normal and abnormal ankle joints: amount and distribution as seen on MR images. AJR Am J Roentgenol 162:111–114
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gursoy, M., Dag, F., Mete, B.D. et al. The anatomic variations of the posterior talofibular ligament associated with os trigonum and pathologies of related structures. Surg Radiol Anat 37, 955–962 (2015). https://doi.org/10.1007/s00276-015-1428-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-015-1428-5